FERIVAFA- ferrous bisglycinate, ascorbic acid, folic acid, cyanocobalamin, biotin, cupric oxide and docusate sodium capsule, gelatin coated
Avion Pharmaceuticals, LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
FeRivaFAFA ™
DESCRIPTION: FeRivaFA ™ is a prescription iron supplement. FeRivaFA™ is a red gel capsule.
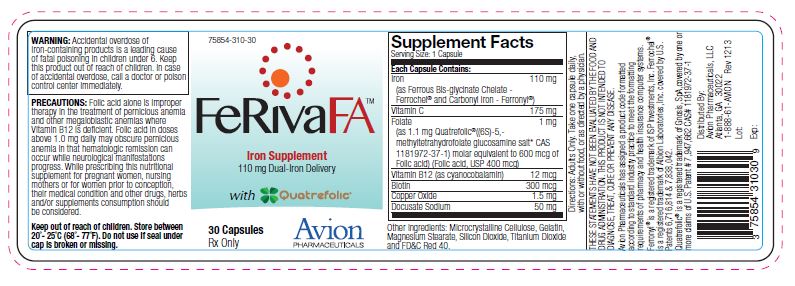
| Supplement Facts
Serving Size: 1 Capsule | |
| Each Capsule Contains: | |
| Iron
(as Ferrous Bis-glycinate Chelate - Ferrochel ®and Carbonyl Iron - Ferronyl ®) | 110 mg |
| Vitamin C | 175 mg |
| Folate
(as 1.1 mg Quatrefolic ®((6S)-5,- methyltetrahydrofolate glucosamine salt* CAS 1181972-37-1) molar equivalent to 600 mcg of Folic acid) (Folic acid, USP 400 mcg) | 1 mg |
| Vitamin B12
(as cyanocobalamin) | 12 mcg |
| Copper Oxide | 1.5 mg |
| Docusate Sodium | 50 mg |
OTHER INGREDIENTS: Microcrystalline Cellulose, Gelatin, Magnesium Stearate, Silicon Dioxide, Titanium Dioxide and FD&C Red 40.
INDICATIONS: FeRivaFA ™ is a multivitamin/multimineral dietary supplement indicated for use in improving the nutritional status of patients with iron deficiency.
CONTRAINDICATIONS: FeRivaFA ™ is contraindicated in patients with a known hypersensitivity to any of the ingredients. Hemochromatosis and hemosiderosis are contraindicated to iron therapy.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
PRECAUTIONS: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid in doses above 1.0 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. While prescribing this nutritional supplement for pregnant women, nursing mothers or for women prior to conception, their medical condition and other drugs, herbs and/or supplements consumption should be considered.
ADVERSE REACTIONS: Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
DOSAGE AND ADMINISTRATION: One capsule daily with or without food or as prescribed by your healthcare provider.
HOW SUPPLIED: In child-resistant bottles of 30 capsules (75854-310-30) and as a sample in a 3-dose unit pack (75854-310-03). The listed product numbers are not National Drug Codes, but have instead been formatted to comply with standard industry practice for pharmacy computer systems.
MANUFACTURED FOR:
Avion Pharmaceuticals, LLC
Atlanta, GA 30022
1-888-61-AVION
Ferronyl ® is a registered trademark of ISP Investments, Inc. Ferrochel ® is a registered trademark of Albion Laboratories, Inc. covered by U.S. Patents 6,716,814 & 7,838,042. Quatrefolic ® is a registered trademark of Gnosis, SpA,covered by one or more claims of U.S. Patent # 7,947,662 CAS# 1181972-37-1
Rev. 0114
THESE STATEMENTS HAVE NOT BEEN EVALUATED BY THE FOOD AND DRUG ADMINISTRATION. THIS PRODUCT IS NOT INTENDED TO DIAGNOSE, TREAT, CURE OR PREVENT ANY DISEASE.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
| FERIVAFA
ferrous bisglycinate, ascorbic acid, folic acid, cyanocobalamin, biotin, cupric oxide and docusate sodium capsule, gelatin coated |
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Labeler - Avion Pharmaceuticals, LLC (965450542) |