Inactive Ingredients
Benzalkonium Chloride 0.013% as preservative, Calcium Chloride Dihydrate, Magnesium Chloride Hexahydrate, Potassium Chloride, Sodium Acetate Trihydrate, Sodium Chloride, Sodium Citrate Dihydrate, Sodium Hydroxide and/or Hydrochloric Acid to adjust pH. The pH range of solution is in the physiologic range.
Uses
For irrigating the eye to help relieve irritation, discomfort and burning by removing loose foreign material, air pollutants (smog or pollen), or chlorinated water.
Warnings
For external use only
Obtain immediate medical treatment for all open wounds in or near the eyes.
This product is not to be used as a saline solution for rinsing and soaking soft contact lenses.
NOT FOR INJECTION OR INTRAOCULAR SURGERY.
Do not use
- if solution changes color or becomes cloudy.
- if you are sensitive to any ingredient in this product.
When using this product
- remove contact lenses before using.
- to avoid contamination, do not touch tip of container to any surface.
- replace cap after using.
Stop use and ask a doctor if you experience
- eye pain
- changes in vision
- continued redness
- irritation of the eye
- if the condition worsens or persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Uses
For irrigating the eye to help relieve irritation, discomfort and burning by removing loose foreign material, air pollutants, (smog or pollen), or chlorinated water.
Warnings
For external use only
Obtain immediate medical treatment for all open wounds in or near the eyes.
This product is not to be used as a saline solution for rinsing and soaking soft contact lenses.
NOT FOR INJECTION OR INTRAOCULAR SURGERY.
Do not use
- if this solution changes color or becomes cloudy.
- if you are sensitive to any ingredient in this product.
When using this product
- remove contact lenses before using.
- to avoid contamination, do not touch tip of container to any surface.
- replace cap after using
Stop use and ask a doctor if you experience
- eye pain
- changes in vision
- continued redness
- irritation of the eye
- if the condition worsens or persists for more than 72 hours
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Flush the affected eye as needed, controlling the rate of flow of solution by pressure on the bottle.
Inactive Ingredients
Benzalkonium Chloride 0.013% as a preservative, Calcium Chloride Dihydrate, Magnesium Chloride Hexahydrate, Potassium Chloride, Sodium Acetate Trihydrate, Sodium Citrate Dihydrate, Sodium Chloride and Sodium Hydroxide and/or Hydrochloric Acid to adjust pH. The pH of the solution is in the physiological range.
PRINCIPAL DISPLAY PANEL
NDC 0065-0530-01
eye
stream®
eye wash
solution
sterile
Alcon
1 FL OZ (30 mL)
EYE-STREAM® eye wash
solution is a sterile and stable
solution that is specially
designed and packaged for use
in the eye(s). Formulated as a
buffered salt solution, it closely
approximates normal human
tear fluid.
Please read this carton
carefully and keep for future
reference.
TAMPER EVIDENT: For your
protection, this bottle has an
imprinted seal around the neck.
Do not use if seal is damaged
or missing at time of purchase.
Alcon
ALCON LABORATORIES, INC.
Fort Worth, Texas 76134 USA
Printed in USA
©2000, 2003, 2010 Alcon, Inc.
300047379-0321
LOT: EXP:

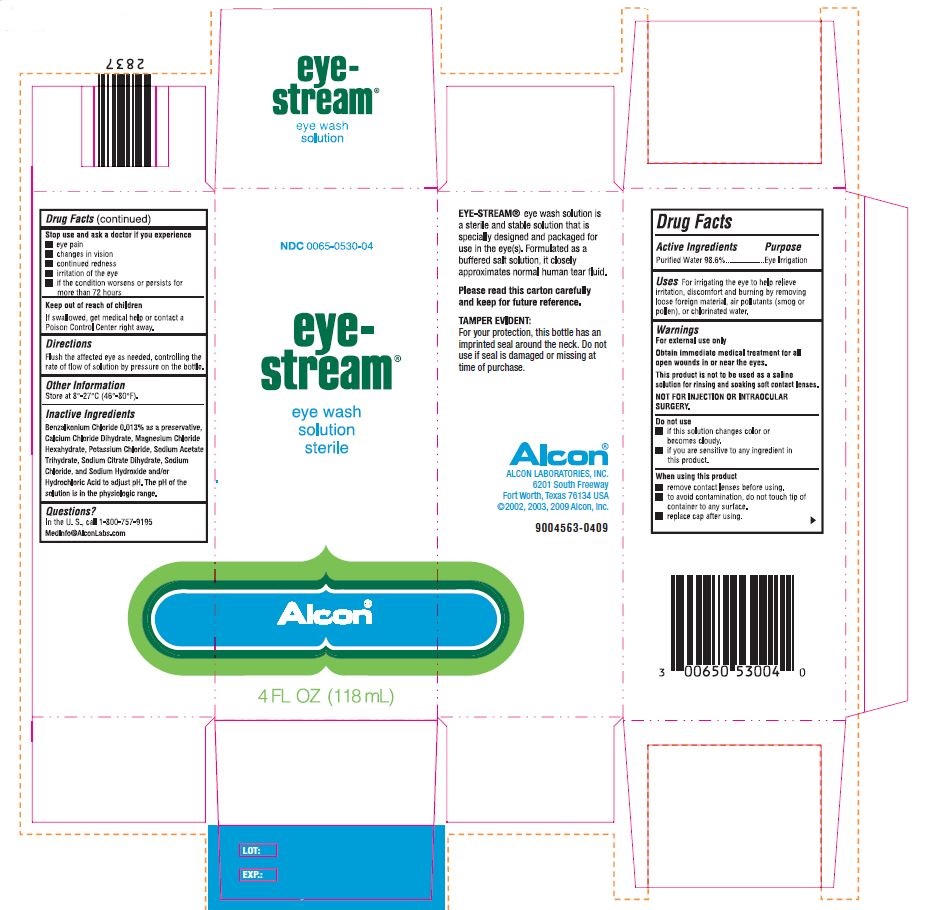
PRINCIPAL DISPLAY PANEL
NDC 0065-0530-04
eye-
stream®
eye wash
solution
sterile
Alcon
4 FL OZ (118 mL)
EYE-STREAM® eye wash solution is
a sterile and stable solution that is
specially designed and packaged for
use in the eye(s). Formulated as a
buffered salt solution, it closely
approximates normal human tear fluid.
Please read this carton carefully
and keep for future reference.
TAMPER EVIDENT:
For your protection, this bottle has an
imprinted seal around the neck. Do not
use if seal is damaged or missing at
time of purchase.
Alcon©
ALCON LABORATORIES, INC.
6201 South Freeway
Fort Worth, Texas 76134 USA
© 2000, 2003, 2009 Alcon, Inc.
9004563-0409
LOT:
EXP: