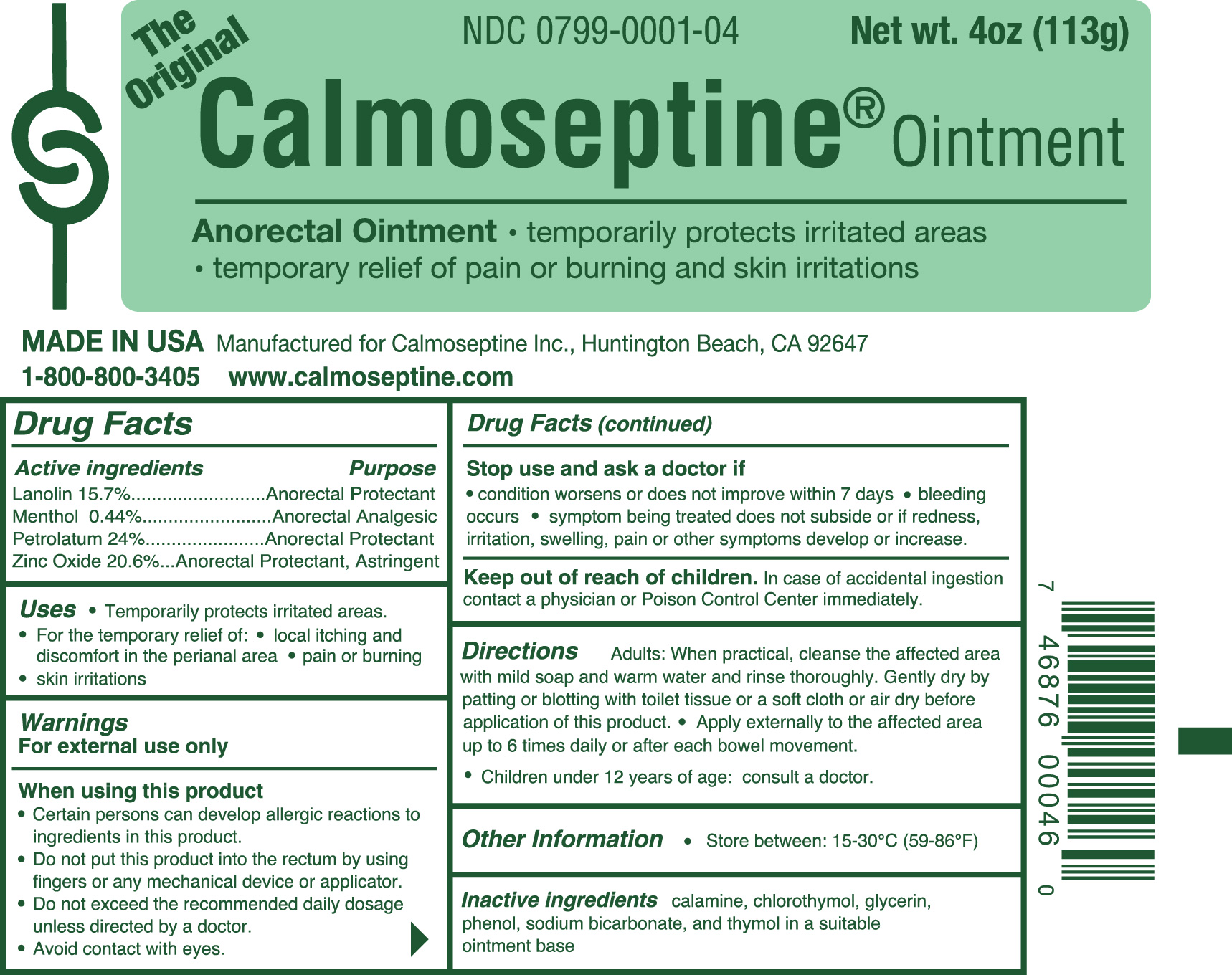
Uses
- Temporarily protects irritated areas.
- For the temporary relief of:
- local itching and discomfort in the perianal area
- pain or burning
- skin irritations
Warnings
For external use only
When using this product
* Certain persons can develop allergic reactions to ingredients in this product.
* Do not put this product into the rectum by using fingers or any mechanical device or applicator.
* Do not exceed the recommended daily dosage unless directed by a doctor.
* Avoid contact with eyes.
Stop use and ask doctor if
- condition worsens or does not improve within 7 days
- bleeding occurs
- symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase.
Keep out of reach of children.
In case of accidental ingestion contact a physician or Poison Control Center immediately.
Directions
Adults: When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly.
Gently dry by patting or blotting with toilet tissue or a soft cloth or air dry before application of this product.
Apply externally to the affected area up to 6 times daily or after each bowel movement.
Children under 12 years of age: consult a doctor.