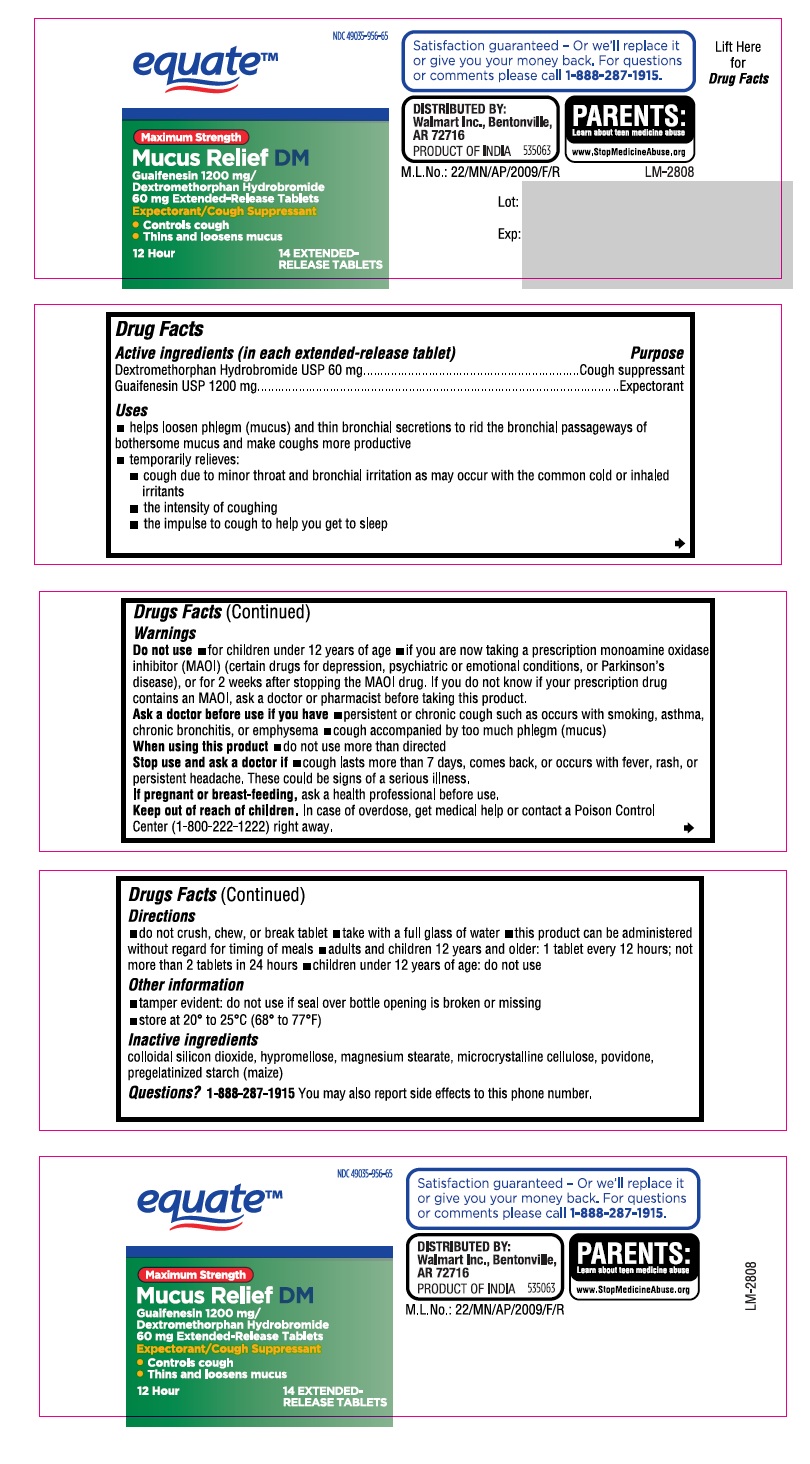
Active ingredients
(in each extended-release tablet)
Dextromethorphan Hydrobromide USP 60 mg
Guaifenesin USP 1200 mg
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help you get to sleep
Warnings
Do not use
- for children under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Stop use and ask a doctor if
- cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for timing of meals
- adults and children 12 years and older: 1 tablet every 12 hours; not more than 2 tablets in 24 hours
- children under 12 years of age: do not use
Other information
- tamper evident: do not use if seal over bottle opening is broken or missing
- store at 20° to 25°C (68° to 77°F)
Inactive ingredients
colloidal silicon dioxide, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch (maize)
Questions?
call 1-888-287-1915 You may also report side effects to this phone number.
DISTRIBUTED BY: Walmart Inc.,
Bentonville, AR 72716
PRODUCT OF INDIA
'This Product is not manufactured or distributed by Reckitt Benckiser, owner of the registered trademark
Maximum Strength Mucinex® DM.
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1200 mg/60 mg (14 Tablet Carton Label)
NDC 49035-956-65
equateTM
Compare to Maximum Strength
Mucinex® DM active ingredients*
Maximum Strength
Mucus Relief DM
Guaifenesin 1200 mg / Dextromethorphan Hydrobromide 60 mg
Extended-Release Tablets
Expectorant/Cough Suppressant
12 Hour
- Controls cough
- Thins and loosens mucus
14 EXTENDED-RELEASE
TABLETS
63

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1200 mg/60 mg (14 Tablet bottle)
NDC 49035-956-65
equateTM
Maximum Strength
Mucus Relief DM
Guaifenesin 1200 mg / Dextromethorphan Hydrobromide 60 mg
Extended-Release Tablets
Expectorant/Cough Suppressant
- Controls cough
- Thins and loosens mucus
12 Hour
14 EXTENDED-RELEASE
TABLETS