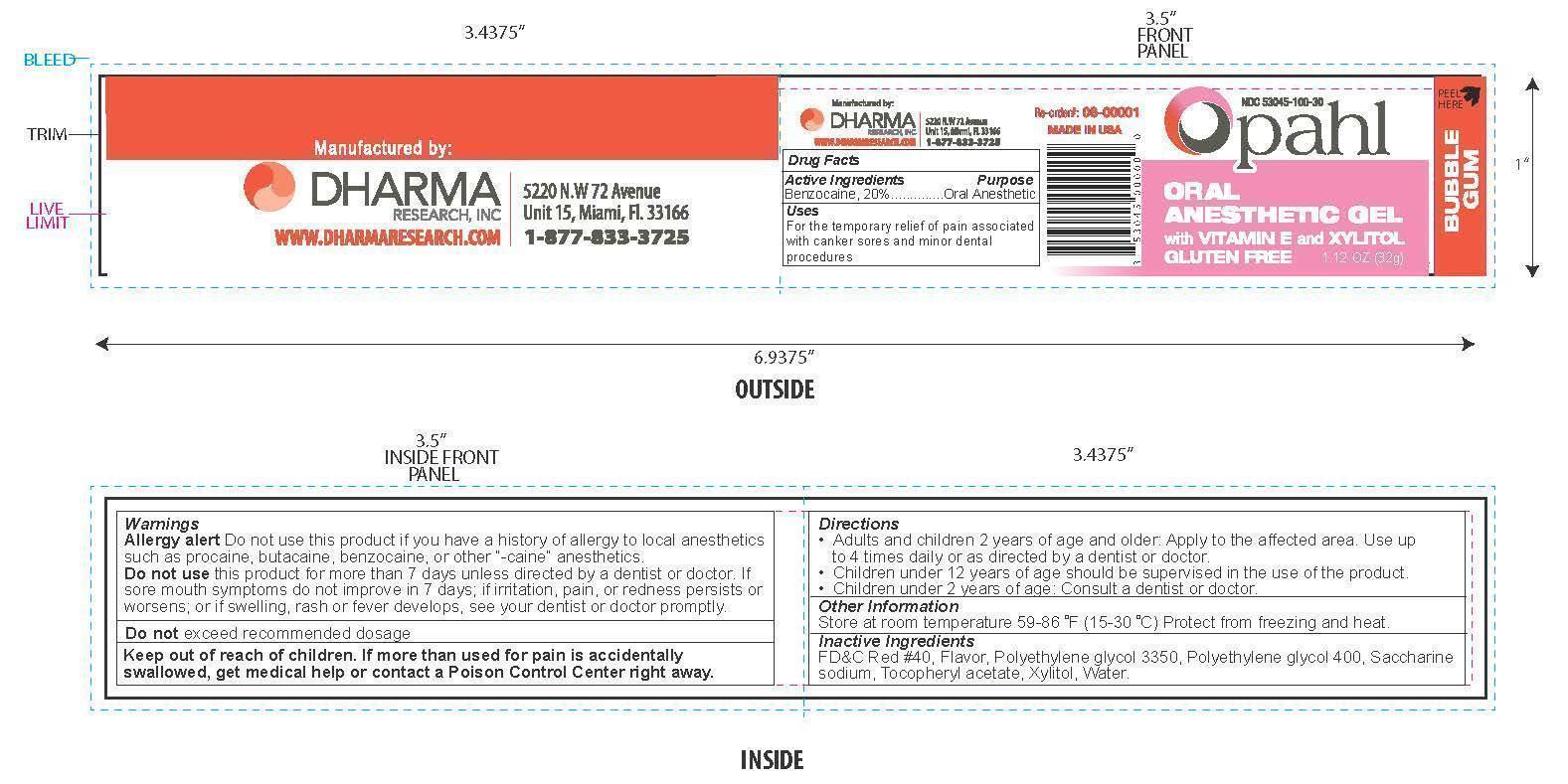
OPAHL- benzocaine gel
Dharma Research, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
Allergy alert Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "-caine" anesthetics.
Keep out of reach of children
If more than used for pain is accidentially swallowed, get medical help or contact a Poison Control center right away.
Directions
- Adults and children 2 years of age and older: Apply to the affected area. Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age should be supervised in the use of this product
- Children under 2 years of age: Consult a dentist or doctor.
Inactive Ingredients
FD&C Red #40, Flavor, Polyethylene glycol 3350, Polyethylene glycol 400, Saccharine sodium, Tocopheryl acetate, Xylitol, Water
| OPAHL
benzocaine gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Dharma Research, Inc. (078444642) |
| Registrant - Dharma Research, Inc. (078444642) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dharma Research, Inc. | 078444642 | manufacture(53045-100) | |
Revised: 10/2018
Document Id: 05f924ad-e4ca-4692-a358-2d018d618fcf
Set id: fedc2da3-ccf0-494c-8650-45934a203f7f
Version: 7
Effective Time: 20181014
Dharma Research, Inc.