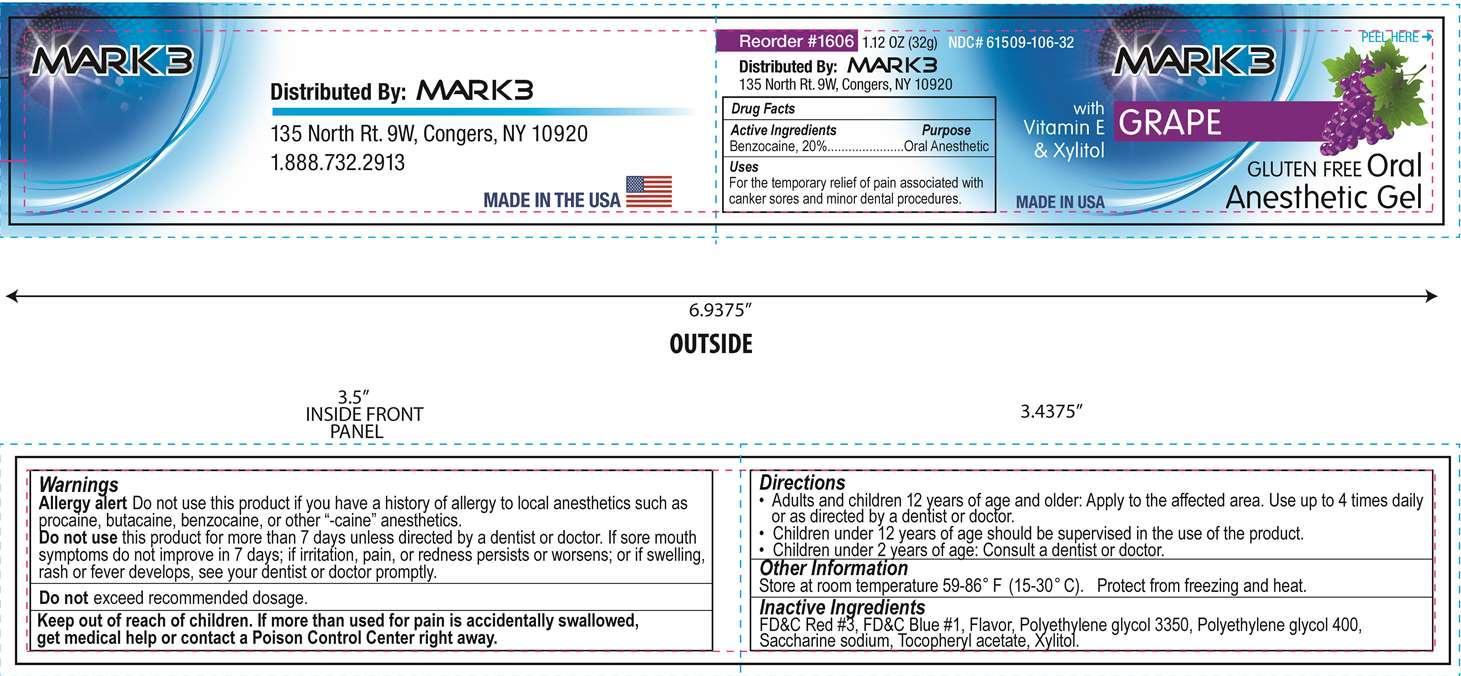
MARK 3- benzocaine gel
Cargus International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Benzocaine, 20%
Uses
For the temporary relief of pain associated with canker sores and minor dental procedures.
Warnings
Allergy alert Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or other "-caine" anesthetics.
Do not
use this product for more than 7 days unless directed by a dentist or doctor. If sore mouth symptoms do not improve in 7 days; if irritation, pain, or redness persists or worsens; or if swelling, rash or fever develops, see your dentist or doctor promptly
Do not
exceed recommended dosage.
Keep out of reach of children
If more than used for pain is accidentially swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 12 years of age and older: Apply to the affected area. Use up to 4 times daily or as directed by a dentist or doctor.
- Children under 12 years of age should be supervised in the use of the product.
- Children under 2 years of age: Consult a dentist or doctor
Other Information
Store at room temperature 59 - 86°F (15 - 30°C). Protect from freezing and heat.
Inactive Ingredients
FD&C Red No. 3, FD&C Blue No. 1, Flavor, Polyethylene glycol 3350, Polyethylene glycol 400, Saccharine sodium, Tocopheryl acetate, Xylitol
Distributed by
Mark 3, 135 North Rt. 9W, Congers, NY 10920
1.888.732.2913
Mark 3
Oral Anesthetic Gel
Grape
Gluten Free
with Vitamin E and Xylitol
NDC 61509-106-32
Net Wt. 1.12 oz. (32 g)
Distributed by Mark 3, 135 North Rt. 9W, Congers, NY 10920
Made in USA
Cargus International, Inc.
