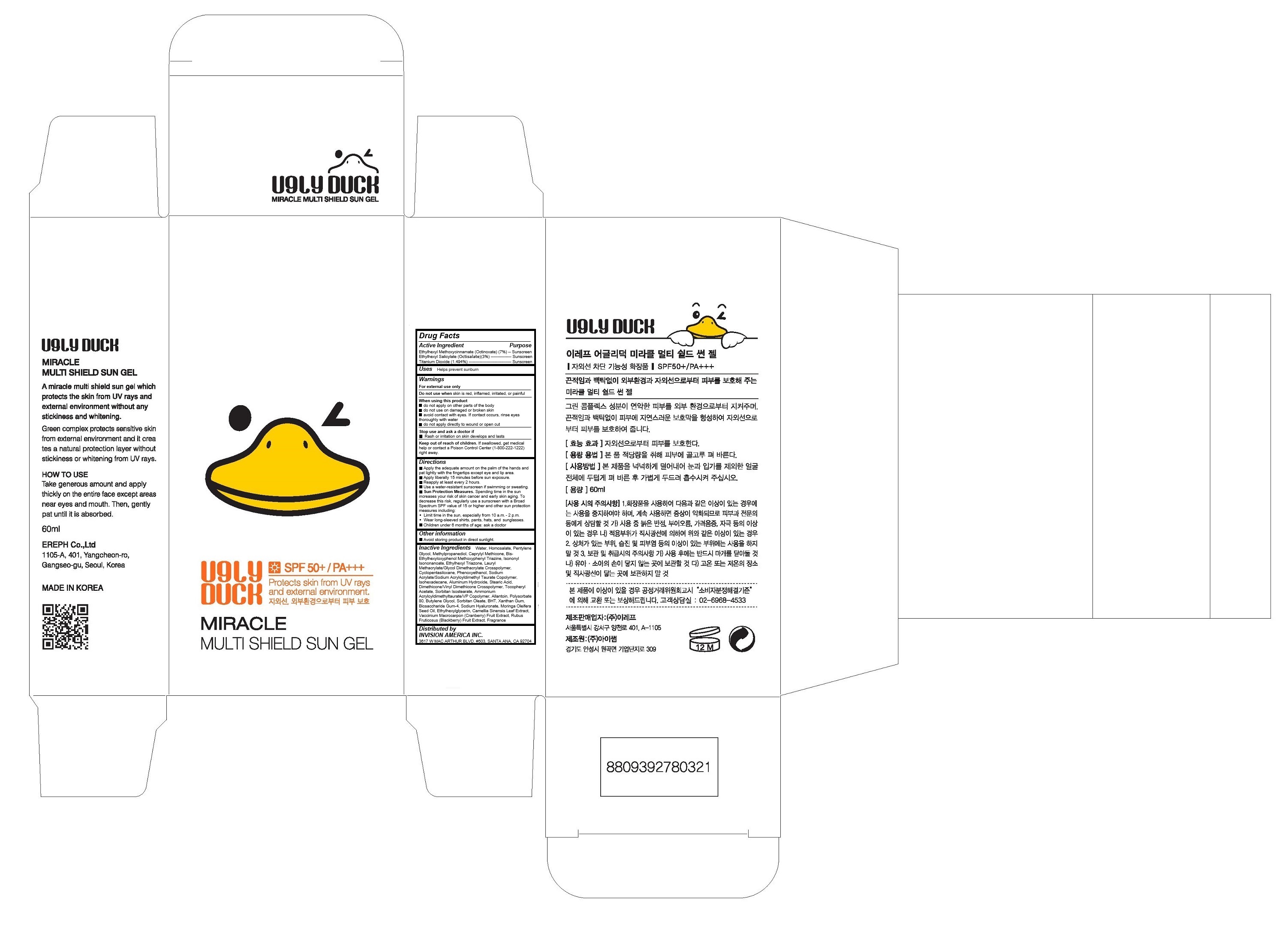
Active Ingredients
Ethylhexyl Methoxycinnamate (Octinoxate) (7%)
Ethylhexyl Salicylate (Octisalate)(3%)
Titanium Dioxide (1.494%)
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Warnings
For external use only
Do not use when skin is red, inflamed, irritated, or painful
When using this product
- do not apply on other parts of the body
- do not use on damaged or broken skin
- avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
- do not apply directly to wound or open cut
Stop use and ask a doctor if
- Rash or irritation on skin develops and lasts
Directions
• Apply liberally 15 minutes before sun exposure.
• Reapply at least every 2 hours.
• Use a water-resistant sunscreen if swimming or sweating.
• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, especially from 10 a.m. - 2 p.m.
Wear long-sleeved shirts, pants, hats, and sunglasses.
Children under 6 months of age: ask a doctor
Inactive Ingredients
Water, Homosalate, Pentylene Glycol, Methylpropanediol, Caprylyl Methicone, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Isononyl Isononanoate, Ethylhexyl Triazone, Lauryl Methacrylate/Glycol Dimethacrylate Crosspolymer, Cyclopentasiloxane, Phenoxyethanol, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Aluminum Hydroxide, Stearic Acid, Dimethicone/Vinyl Dimethicone Crosspolymer, Tocopheryl Acetate, Sorbitan Isostearate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Allantoin, Polysorbate 80, Butylene Glycol, Sorbitan Oleate, BHT, Xanthan Gum, Biosaccharide Gum-4, Sodium Hyaluronate, Moringa Oleifera Seed Oil, Ethylhexylglycerin, Camellia Sinensis Leaf Extract, Vaccinium Macrocarpon (Cranberry) Fruit Extract,