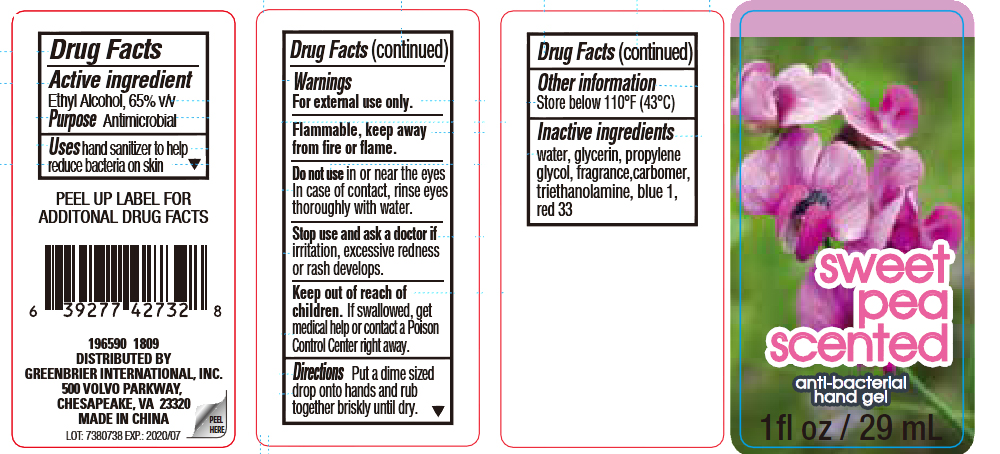
SWEET PEA SCENTED ANTIMICROBIAL HAND GEL- ethyl alcohol liquid
Nantong Health & Beyond Hygienic Products Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Ethyl Alcohol, 65% v/v
Uses hand sanitizer to help
reduce bacteria on skin
Warnings
For external use only.
Flammable, keep away from
fire or flame.
Do not use in or near the eyes
In case of contact, rinse eyes
thoroughly with water.
Stop use and ask a doctor if
irritation, excessive redness
or rash develops.
Keep out of reach of
children. If swallowed, get
medical help or contact a Poison
Control Center right away.
Directions Put a dime sized
drop onto hands and rub
together briskly until dry.
Other information
Store below 110° F (43° C)
Inactive ingredients
water, glycerin, propylene
glycol, fragrance, carbomer,
triethanolamine, blue 1,
red 33
Nantong Health & Beyond Hygienic Products Inc.