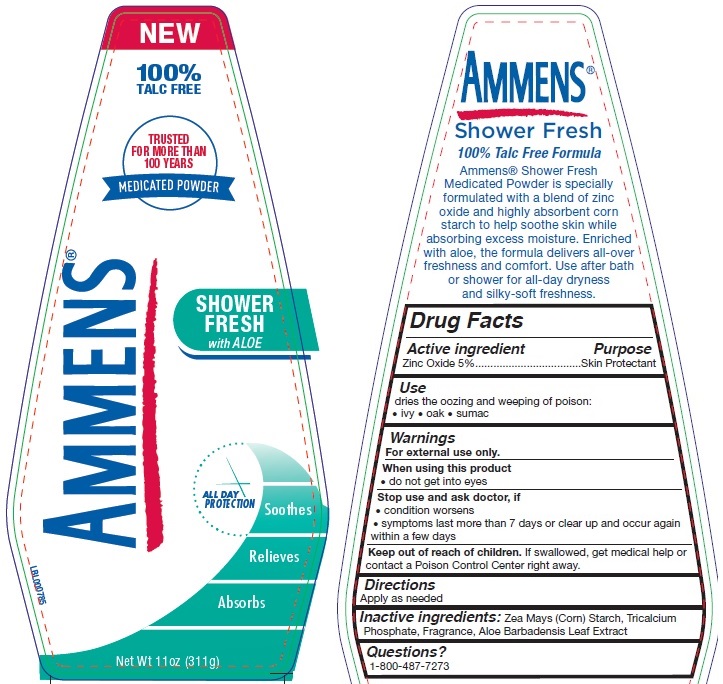
AMMENS MEDICATED POWDER SHOWER FRESH- zinc oxide powder
Idelle Labs, Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Zinc Oxide 5%
Use
dries the oozing and weeping of poison:
• ivy
• oak
• sumac
Warnings
For external use only.
When using this product
• do not get into eyes
Stop use and ask doctor, if
• condition worsens
• symptoms last more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply as needed
Inactive ingredients:
Zea Mays (Corn) Starch, Tricalcium Phosphate, Fragrance, Aloe Barbadensis Leaf Extract
Questions?
call to 1-800-487-7273
©IDELLE LABS, LTD.
All rights reserved.
Distributed by Idelle Labs,
Ltd., El Paso, TX 79912
Ammens® is a trademark
of Kaz Europe Sarl.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
AMMENS®
NEW 100% TALC FREE
TRUSTED FOR MORE THAN 100 YEARS
MEDICATED POWDER
SHOWER FRESH with ALOE
Soothes
Relieves
Absorbs
Net Wt 11oz (311g)