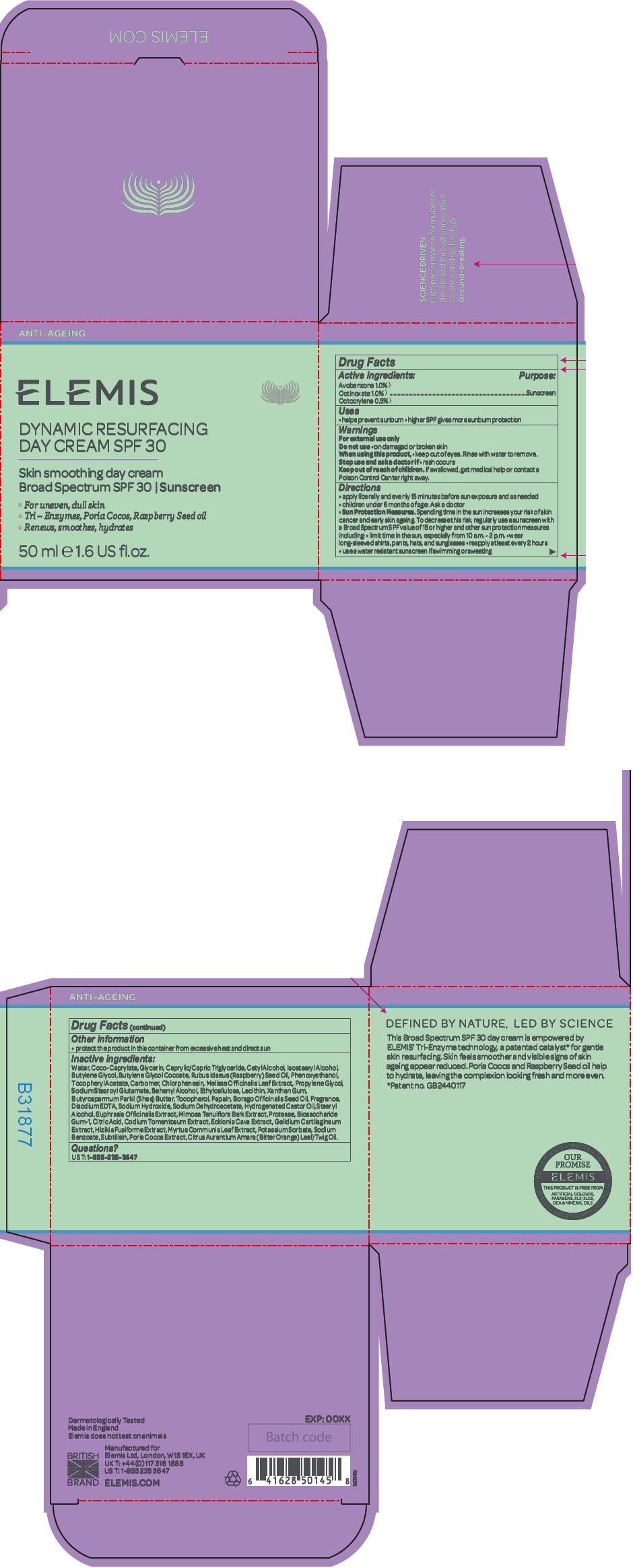
Directions
- apply liberally and evenly 15 minutes before sun exposure and as needed
- children under 6 months of age: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin ageing. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
Inactive ingredients
Water, Coco-Caprylate, Glycerin, Caprylic/Capric Triglyceride, Cetyl Alcohol, Isostearyl Alcohol, Butylene Glycol, Butylene Glycol Cocoate, Rubus Idaeus (Raspberry) Seed Oil, Phenoxyethanol, Tocopheryl Acetate, Carbomer, Chlorphenesin, Melissa Officinalis Leaf Extract, Propylene Glycol, Sodium Stearoyl Glutamate, Behenyl Alcohol, Ethylcellulose, Lecithin, Xanthan Gum, Butyrospermum Parkii (Shea) Butter, Tocopherol, Papain, Borago Officinalis Seed Oil, Fragrance, Disodium EDTA, Sodium Hydroxide, Sodium Dehydroacetate, Hydrogenated Castor Oil, Stearyl Alcohol, Euphrasia Officinalis Extract, Mimosa Tenuiflora Bark Extract, Protease, Biosaccharide Gum-1, Citric Acid, Codium Tomentosum Extract, Ecklonia Cava Extract, Gelidium Cartilagineum Extract, Hizikia Fusiforme Extract, Myrtus Communis Leaf Extract, Potassium Sorbate, Sodium Benzoate, Subtilisin, Poria Cocos Extract, Citrus Aurantium Amara (Bitter Orange) Leaf/Twig Oil.