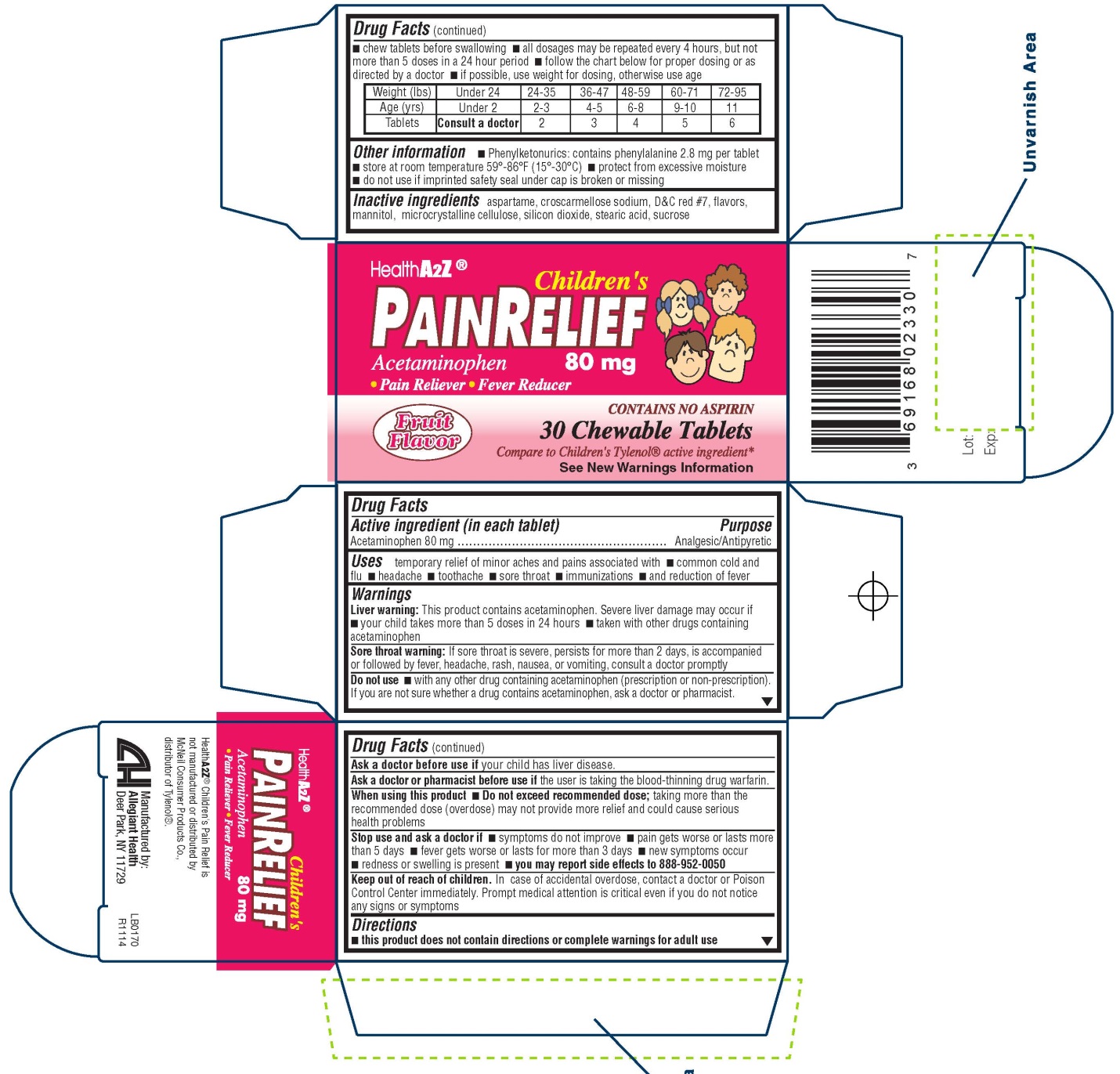
Uses
temporary relief of minor aches and pains associated with common cold and flu headache toothache sore throat immunizations and reduction of fever
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if your child takes more than 5 doses in 24 hours taken with other drugs containing acetaminophen
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly
Do not use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
Keep Out of Reach of Children
In case of accidental overdose, contact a doctor or Poison Control Center immediately. Prompt medical attention is critical even if you do not notice any signs or symptoms
Directions
this product does not contain directions or complete warnings for adult usechew tablets before swallowing all dosages may be repeated every 4 hours, but not more than 5 doses in a 24 hour period follow the chart below for proper dosing or as directed by a doctor if possible, use weight for dosing, otherwise use age
|
Weight (lbs) |
Under 24 |
24-35 |
36-47 |
48-59 |
60-71 |
72-95 |
|
Age (yrs) |
Under 2 |
2-3 |
4-5 |
6-8 |
9-10 |
11 |
|
Tablets |
Consult a doctor |
2 |
3 |
4 |
5 |
6 |
Other information
Phenylketonurics: contains phenylalanine 2.8 mg per tablet store at room temperature protect from excessive moisture do not use if imprinted safety seal under cap is broken or missing