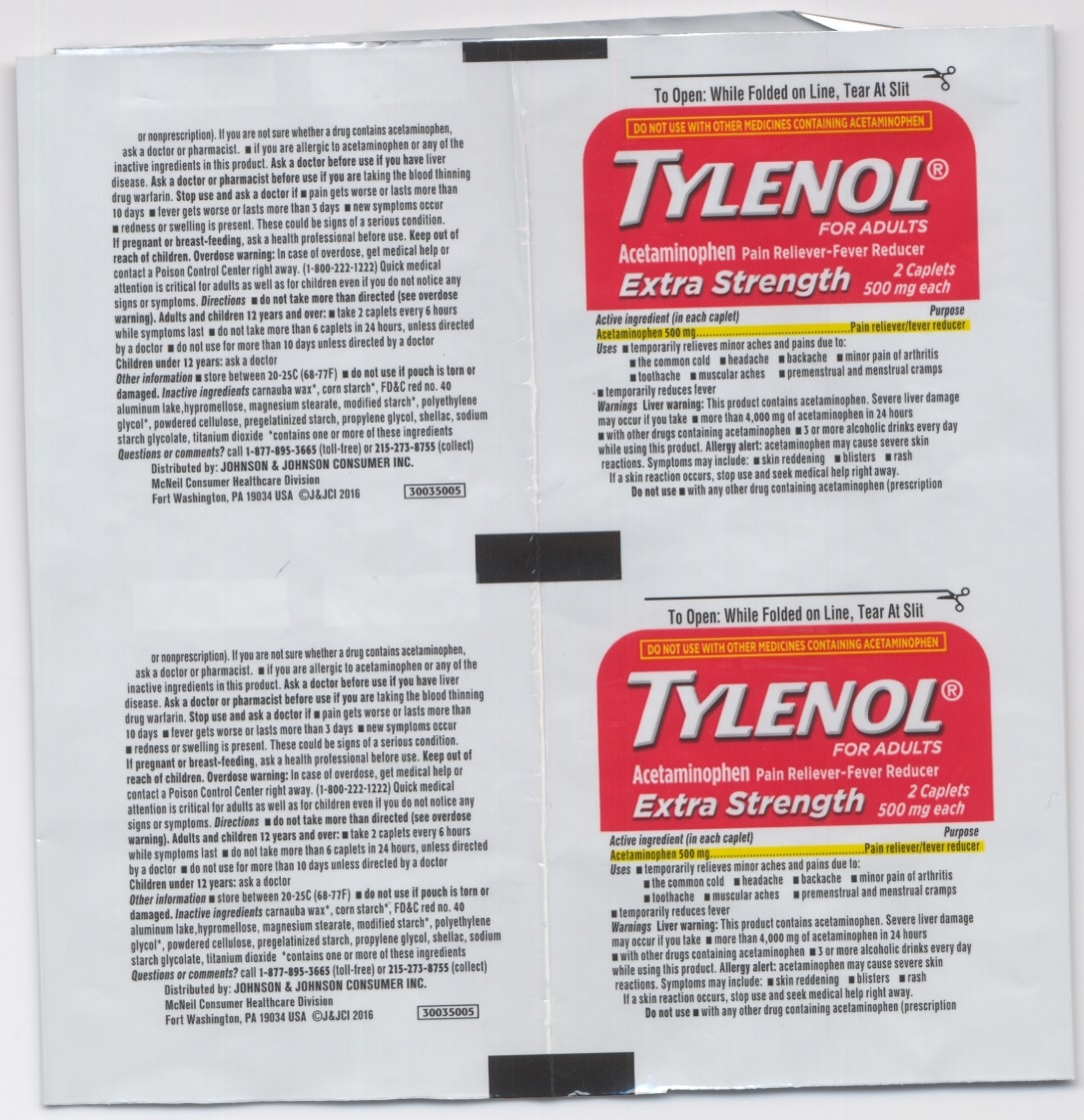
Uses
• temporarily relieves minor aches and pains due to:
• the common cold • headache • backache • minor pain of arthritis • toothache • muscular aches • premenstrual and menstual cramps • temporarily reduces lever
Warnings
This product contains acetaminophen. Severe liver damage may occur if you take • more than 4,000 mg of acetaminophen in 24 hours Liver warning:
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product. Allergy alert: acetaminophen may cause severe skin reactions.symptoms may include:
- skin reddening
- blisters
- rash if a skin reaction occurs. stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen. ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
Directions
- do not take more than directed (see overdose warning).
| Adults and children 12 years and over: |
|
| children under 12 years | ask a doctor |
Inactive ingredients
carnauba wax*, corn starch*, FD&C red no.40 aluminum lake, hypromellose, magnesium stearate, modified starch*, polyethylene glycol*, powdered cellulose, pregelatinized starch, propylene glycol, shellac, sodium starch glycolate, titanium dioxide * contains one or more of these ingredients