Use
- relieves occasional constipation
- this product generally produces bowel movement in 1 to 5 minutes
Warnings
Ask a doctor before use if the child
- has already used a laxative for more than 3 days
- has kidney disease, have heart problems, or is dehydrated
- is on a sodium-restricted diet
- has abdominal pain, nausea, or vomiting
- has a sudden change in bowel habits lasting more than 2 weeks
Ask a doctor or pharmacist before use if the child is taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
When using this product
- do not use more than directed. Serious side effects may occur from excess dosage
- do not use for more than 3 days, without asking a doctor
Directions (or as directed by a doctor)
Single daily dosage (per 24 hours)
Do not use if taking another sodium phosphate product.
Do not use more unless directed by a doctor. See Warnings.
| children 2 to under 12 years of age | Use 1 bottle once daily |
| children under 2 years of age | DO NOT USE |
Other information
- each 59 mL contains sodium 2.2g
- store at 15 to 30°C (59 to 86°F)
- additional liquids by mouth are recommended while using this product
- Tamper Evident: DO NOT USE IF TOP OR BOTTOM FLAP OF CARTON IS TORN OR MISSING.
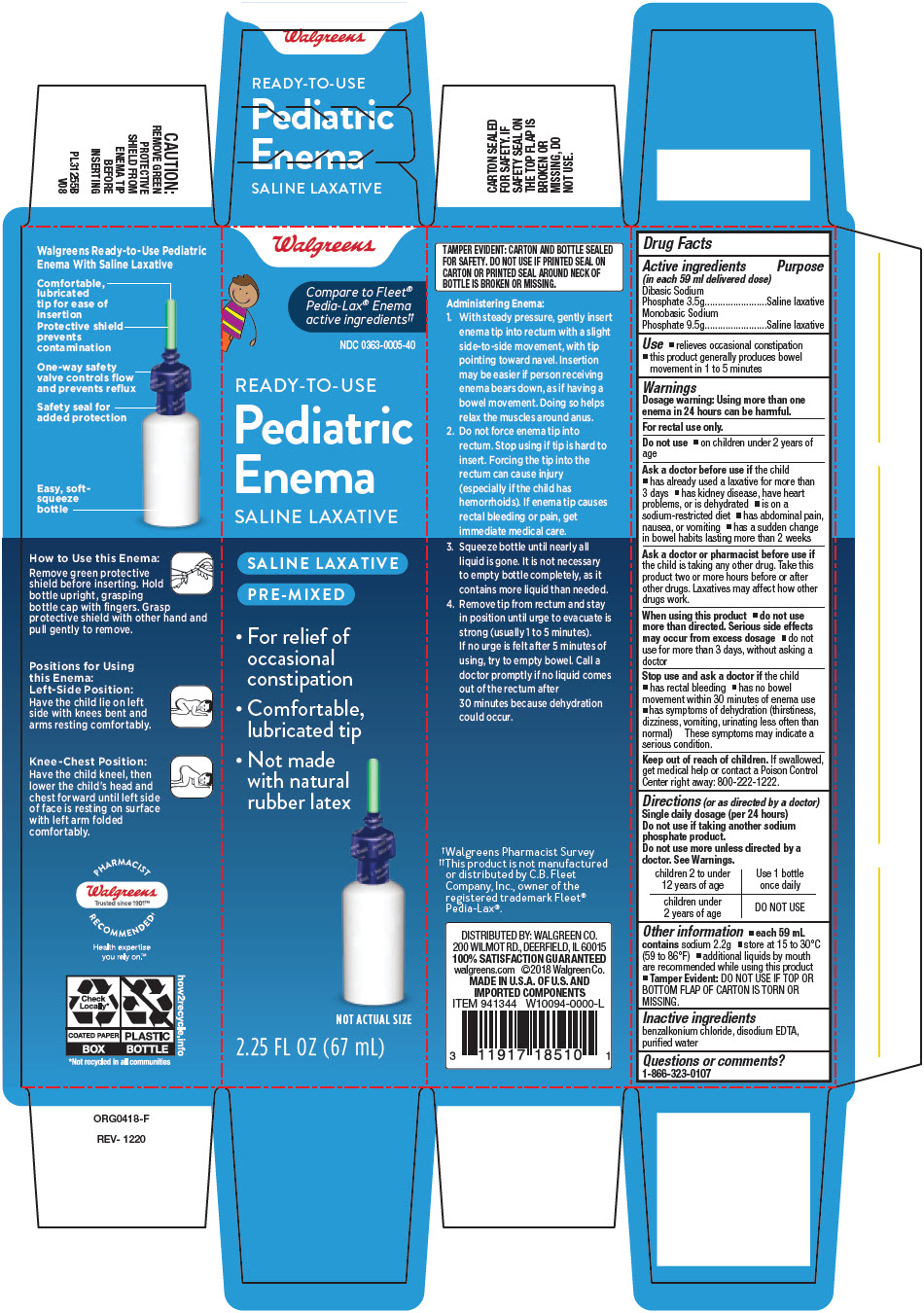
PRINCIPAL DISPLAY PANEL - 67 mL Bottle Box
Walgreens
Compare to Fleet®
Pedia-Lax® Enema
active ingredients††
NDC 0363-0005-40
READY-TO-USE
Pediatric
Enema
SALINE LAXATIVE
SALINE LAXATIVE
PRE-MIXED
- For relief of
occasional
constipation - Comfortable,
lubricated tip - Not made
with natural
rubber latex
NOT ACTUAL SIZE
2.25 FL OZ (67 mL)