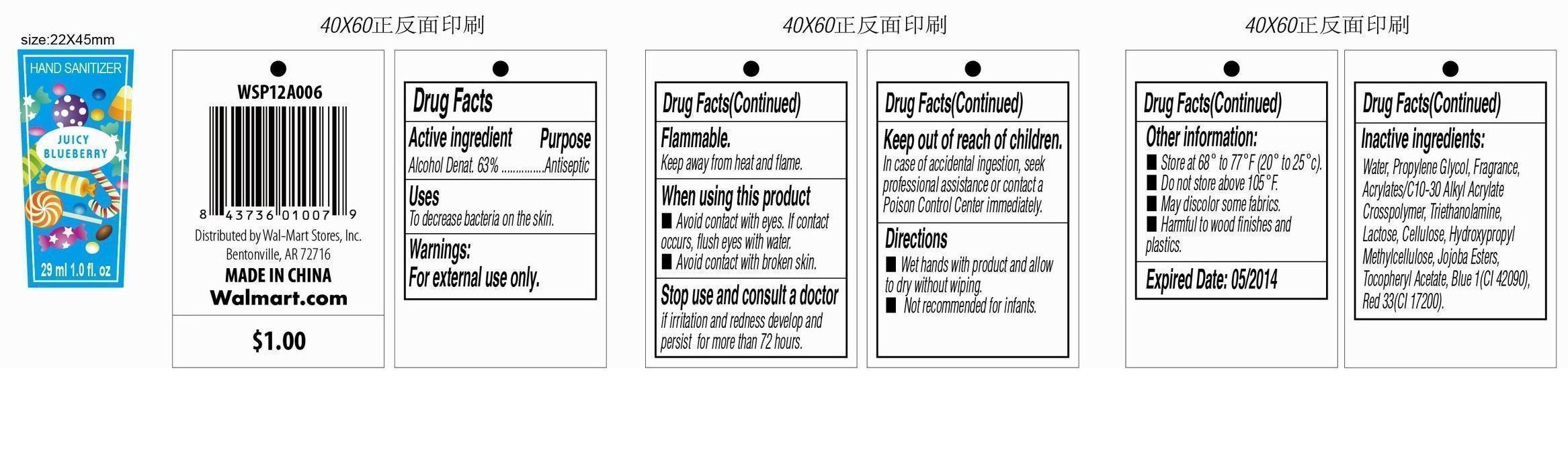
HAND SANITIZER PASSIONFRUIT POMEGRANATE- alcohol liquid
Landy International
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Active Ingredient
Ethyl Alcohol 63%
Purpose
Anticeptic
Uses
To decrease bacteria on the skin.
Warning
For external use only.
Flammable.
Keep away from heat and flame.
When using this product
- Avoid contact with eyes. If contact occurs, flush eyes with water.
- Avoid contact with broken skin.
Stop use and consult a doctor if irritation and reness develop and persis for more than 72 hours.
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
■ Wet hands with product and allow to dry without wiping.
■ Not recommended for infants.
Other Information
- Store at 68 o to 77 oF (20 o to 25 oc).
- Do not store above 105 oF.
- May discolor some fabrics.
- Harmful to wood finishes and plastics.
Inactive Ingredient
Alcohol Denat., Water, Propylene Glycol, Fragrance, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Lactose, Cellulose, Hydroxypropyl Methylcellulose, Jojoba Esters, Tocopheryl Acetate, Blue 1(CI 42090), Red 33(CI 17200), Red 4(CI 14700).