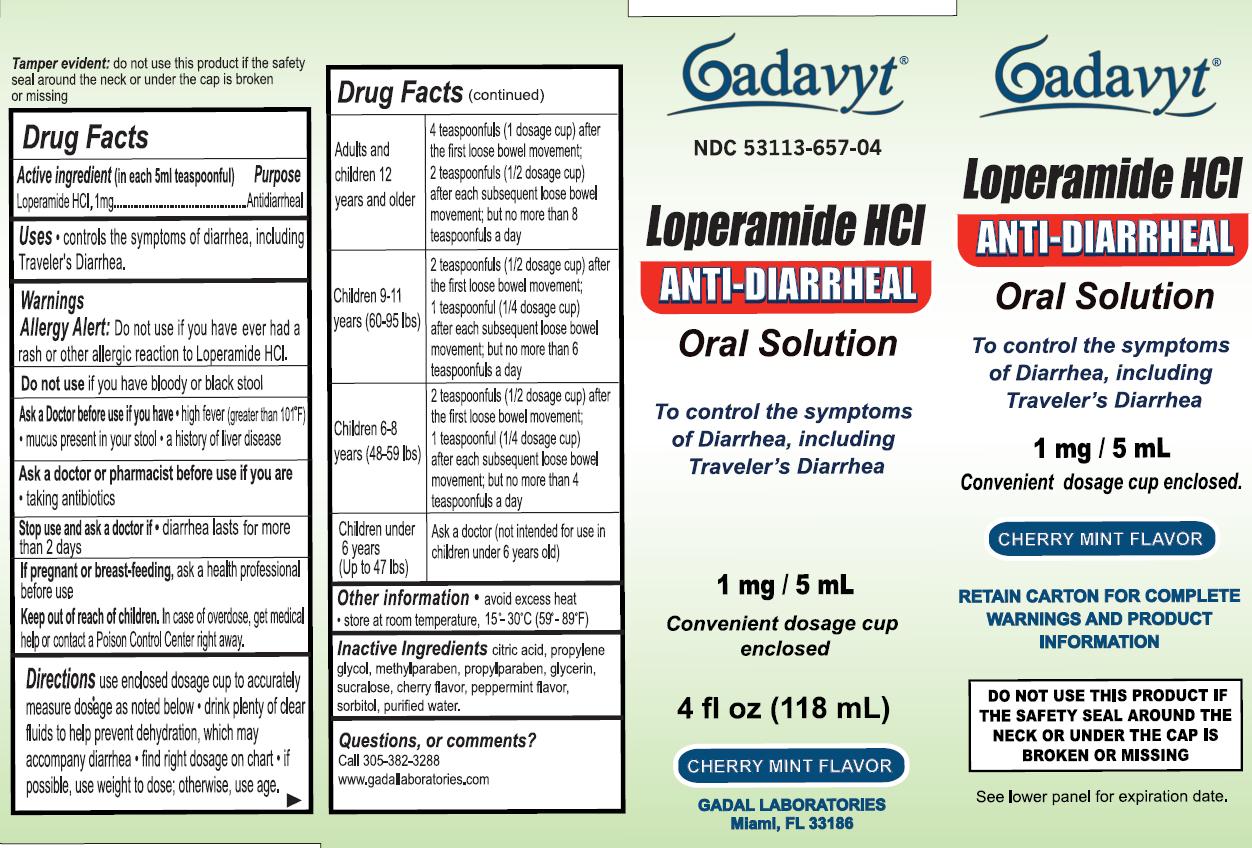
Active Ingredient (in each 5mL teaspoonful) Purpose
Loperamide HCl, 1mg ....................................................Antidiarrheal
Warnings
Allergy Alert:Do not use if you have ever had a rash or other allergic reaction to Loperamide HCl.
Do not use if you have bloody or black stool
Ask a Doctor before use if you have
- high fiver (greater than 101 degrees F)
- mucus present in your stool
- a history of liver disease
Ask a doctor or pharmacist before use if you are taking antibiotics
Keep out of reach of children. In case of overdose, get medical help or contact Poison Control Center right away.
Directions
Use enclosed dosage cup to accurately measure dosage as noted below
- drink plenty of clear fluids to help prevent dehydration, which may accompany diarrhea
- find right dosage on chart
- if possible, use weight to dose; otherwise, use age.
| Adults and children 12 years and older | 4 teaspoonfuls (1 dosage cup) after the first loose bowel movement; 2 teaspoonfuls (1/2 dosage cup) after each subsequent loose bowel movement; but no more than 8 teaspoonfuls a day |
| Children 9-11 (60-95 lbs) | 2 teaspoonfuls (1/2 dosage cup) after the first loose bowel movement; 1 teaspoonful (1/4 dosage cup) after each subsequent loose bowel movement; but no more than 6 teaspoonfuls a day |
| Children 6-8 (48-59 lbs) | 2 teaspoonfuls (1/2 dosage cup) after the first loose bowel movement; 1 teaspoonfuls (1/4 dosage cup) after each subsequent loose bowel movement; but no more than 4 teaspoonfuls a day |
| Children under 6 years (Up to 47 lbs) | Ask a doctor (not intended for use in children under 6 years old |