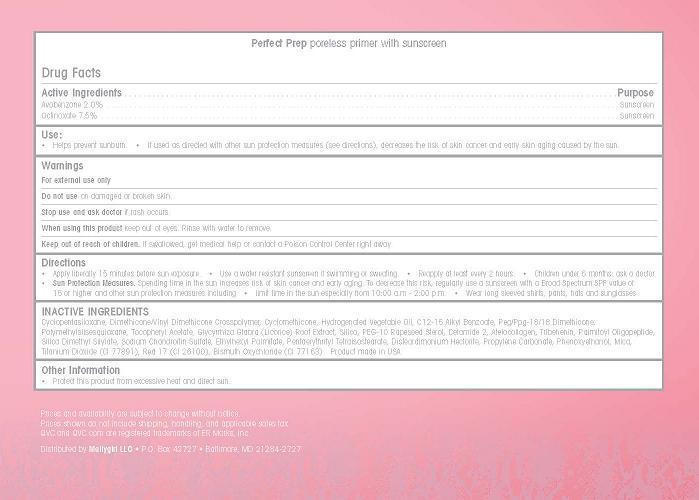
Use
- Helps prevent sunburn
- If used as directed with other sun protection measures (see directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children. If swollowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: ask a doctor
- Sun Protection Measures. Spending time in the sun increases risk of skin cancer and early aging. To decrease this risk regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higer and other sun protection measures including
- Limit time in the sun especially from 10:00 a.m. - 2:00 p.m.
- Wear long sleeved shirts, pants, hats and sunglasses
Inactive Ingredients
Cyclopentasiloxane, Dimethicone/Vinyl Dimeticone Crosspolymer, Cyclomethicone, Hydrogenated Vegetable Oil, C12-15 Alkyl Benzoate, Peg/Ppg-18/18 Dimethicone, Polymethylsilsesquioxane, Tocopheryl Acetate, Glycyrrhiza Glabra (Licorice) Root Extract, Silica, PEG-10 Rapeseed Sterol, Ceramide 2, Atelocollagen, Tribehenin, Palmitoyl Oligopeptide, Silica Dimethyl Silylate, Sodium Chondroitin Sulfate, Pentaerythrityl Tetraisostearate, Ethylthexyl Palmitate, Disteardimonium Hectorite, Propylene Carbonate, Phenoxyethanol, Mica, Titanium Dioxide (CI 77891), Red 17 (CI 26100), Bismuth Oxychloride (CI 77163). Product made in the USA