Uses
- for relief of occasional constipation(irregularity)
- this product generally produces bowel movement in 6 to 12 hours
Warnings
Ask a doctor before use if you have
- abdominal pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
When using this product
- do not chew or crush tablets
- do not take this product within 1 hour after taking an antacid or milk
- abdonimal discomfort,faintness, and cramps may occur
Direction
- each laxative works differently.Adults and children over 12 may need fewer tablets to get the same effect as more tablets of another brand.
- Start with one tablet and take with water.If one tablet does not produce the desired result,then try two or three tablets daily.
- Do not take more than three tablets daily
| adults and children 12 years of age and older | - take 1 to 3 tablets in a single dose once daily |
| children 6 to under 12 years of age | - take 1 tablet once daily |
| children under 6 years of age | - ask a doctor |
Other information
- each tablet contains : calcium 20 mg
- do not exposee to temperatures above 30°C(85°F)
- store between 20° to 25°C (68° to 77° F)
Inactive ingredients
lactose,cornstarch,povidone (K-30),sodium startch glycolate,talc,magnesium stearate,methcylic acidcopolymer,polethylene glaycol,sodium hydroxide,sucrose,acacia,gelatin,methylparaben,propylparaben,calcium sulphate dihydrate,titanium dioxide,D&C red#7 lake, FD & C Red #40; D & C Red # 27
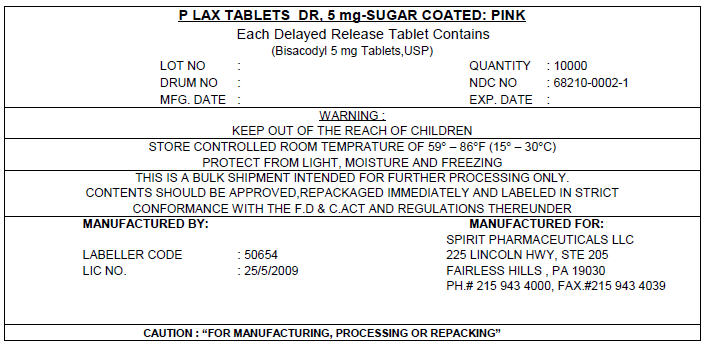
PRINCIPAL DISPLAY PANEL - 5 mg Shipping Label
P LAX TABLETS DR, 5 mg-SUGAR COATED: PINK
Each Delayed Release Tablet Contains
(Bisacodyl 5 mg Tablets,USP)
LOT NO :
DRUM NO :
MFG. DATE :
QUANTITY : 10000
NDC NO : 68210-0002-1
EXP. DATE :
WARNING :
KEEP OUT OF THE REACH OF CHILDREN
STORE CONTROLLED ROOM TEMPRATURE OF 59° – 86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZING
THIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED,REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE F.D & C.ACT AND REGULATIONS THEREUNDER
MANUFACTURED BY:
LABELLER CODE : 50654
LIC NO. : 25/5/2009
MANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS , PA 19030
PH.# 215 943 4000, FAX.#215 943 4039
CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"