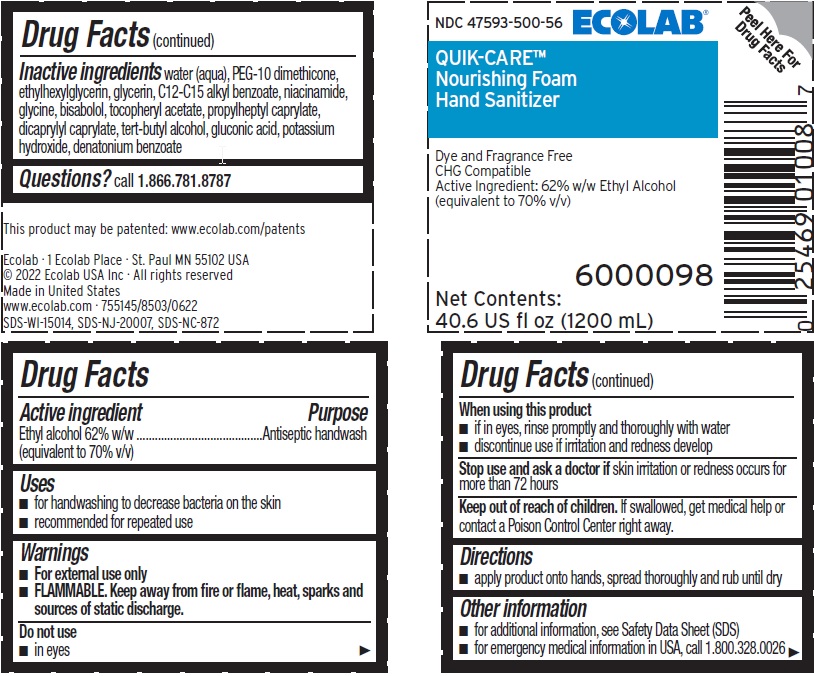
Warnings
- For external use only
- FLAMMABLE, keep away from fire or flame, heat, sparks and sources of static discharge.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Other information
- for additional information, see Safety Data Sheet (MSDS)
- for emergency medical information in USA, call 1 800 328 0026
Inactive ingredients water (aqua), PEG-10 dimethicone, ethylhexylglycerin, glycerin, C12-C15 alkyl benzoate, niacinamide, glycine, bisabolol, tocopheryl acetate, propylheptyl caprylate, dicaprylyl caprylate, tert-butyl alcohol, gluconic acid, potassium hydroxide, denatonium benzoate
Principal Display Panel and Representative Label
ECOLAB®
NDC 47593-500-56
QUIK-CARE®
Nourishing Foam
Hand Sanitizer
Dye and Fragrance Free
CHG Compatible
Active Ingredient: 62% Ethyl Alcohol
(equivalent to 70% v/v)
6000098
Net Contents: 40.6 US fl oz (1200 mL)
This product may be patented: www.ecolab.com/patents
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA
© 2022 Ecolab USA Inc · All rights reserved
Made in United States
www.ecolab.com · 755145/8503/0622
SDS-WI-15014, SDS-NJ-20007, SDS-NC-872