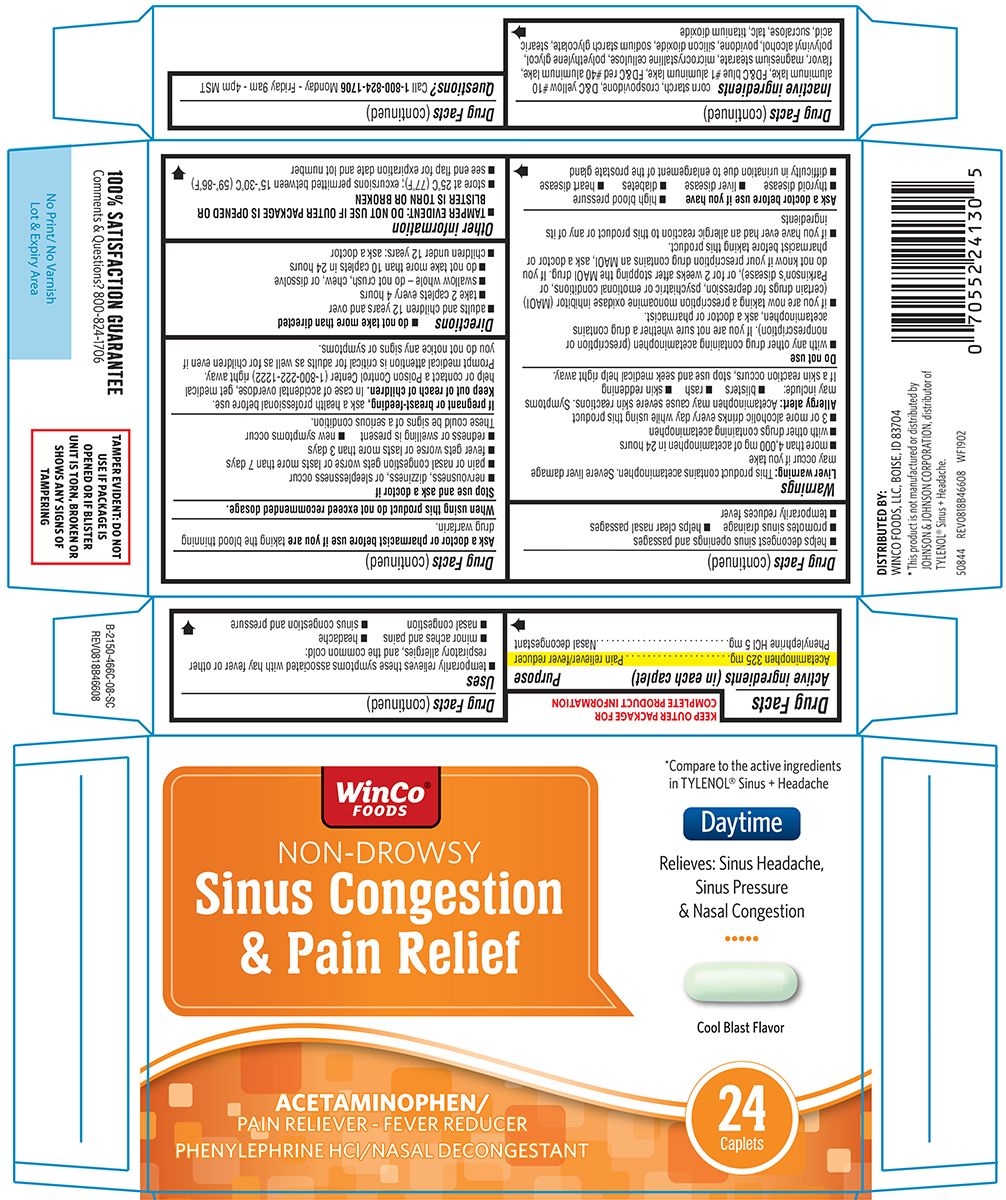
Uses
- temporarily relieves these symptoms associated with hay fever or other respiratory allergies, and the common cold:
- minor aches and pains
- headache
- nasal congestion
- sinus congestion and pressure
- helps decongest sinus openings and passages
- promotes sinus drainage
- helps clear nasal passages
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
- high blood pressure
- thyroid disease
- liver disease
- diabetes
- heart disease
- difficulty in urination due to enlargement of the prostate gland
Directions
-
do not take more than directed
- adults and children 12 years and over
- take 2 caplets every 4 hours
- swallow whole - do not crush, chew, or dissolve
- do not take more than 10 caplets in 24 hours
- children under 12 years: ask a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, crospovidone, D&C yellow #10 aluminum lake, FD&C blue #1 aluminum lake, FD&C red #40 aluminum lake, flavor, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, silicon dioxide, sodium starch glycolate, stearic acid, sucralose, talc, titanium dioxide
Principal Display Panel
WinCo®
FOODS
NON-DROWSY
Sinus Congestion
& Pain Relief
Daytime
*Compare to the active ingredients
in TYLENOL® Sinus + Headache
Relieves: Sinus Headache,
Sinus Pressure
& Nasal Congestion
Cool Blast Flavor
ACETAMINOPHEN/
PAIN RELIEVER - FEVER REDUCER
PHENYLEPHRINE HCl/NASAL DECONGESTANT
24
Caplets
DISTRIBUTED BY:
WINCO FOODS, LLC, BOISE, ID 83704
*This product is not manufactured or distributed by
JOHNSON & JOHNSON CORPORATION, distributor of
TYLENOL® Sinus + Headache.
50844 REV0818B46608 WF1902
100% SATISFACTION GUARANTEE
Comments & Questions? 800-824-1706
TAMPER EVIDENT: DO NOT
USE IF PACKAGE IS
OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR
SHOWS ANY SIGNS OF
TAMPERING
Winco 44-466C