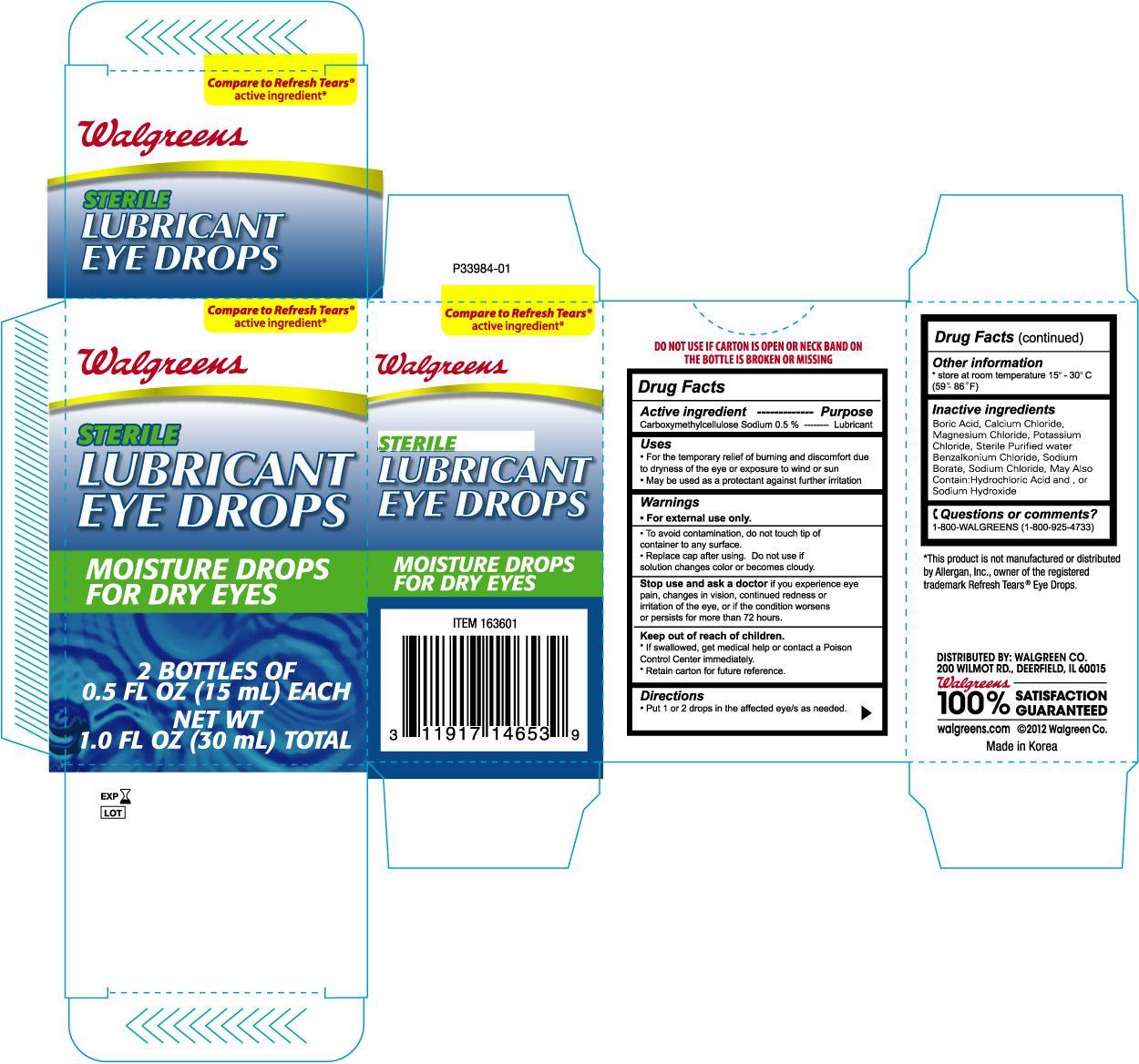
Active ingredient Purpose
Carboxymethylcellulose Sodium 0.5%......................................Lubricant
Uses
- For the temporary relief of burning and discomfort due to dryness of the eye or exposure to wind or sun
- May be used as a protectant against further irritation
To avoid contamination, do not touch tip of container to any surface
Replace cap after using. Do not use if solution changes color or becomes cloudy.
Stop use and ask a doctor if you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours.
Keep out of the reach of children.
- If swallowed, get medical help or contact a Poison Control Center immediately
- Retain carton for future reference.