Your dentist has selected for you FluoriShield, a 1.1% Sodium Fluoride product that was specially developed to prevent tooth decay which can occur from xerostomia (dry mouth). Dry mouth can result from radiation therapy, systemic diseases, medications which affect the salivary glands and mouth breathing. Clinical trials have shown that application of topical fluoride gel in the presence of good oral hygiene can prevent or significantly reduce tooth decay which is related to dry mouth. FluoriShield topical fluoride gel can be applied with a custom carrier or tray, directly brushed on, or used with an over denture. Your dentist will prescribe the method that is best suited for your individual needs. The gel is easily dispensed when the bottle is inverted for storing.
STORE INVERTED FOR EASY REMOVAL OF CONTENTS.
Description:
Topical neutral 1.1% sodium fluoride for use as a dental caries preventative in children and adults. This prescription product is not a dentifrice.
Warnings:
DO NOT SWALLOW. As with all medications, this product should be kept out of reach of children.
INSTRUCTIONS
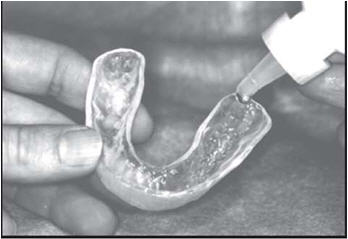
Custom Carriers or Trays
Brush and floss your teeth to remove plaque. Adults and children 6 years of age or older: Add one drop of FluoriShield into each tooth area in the custom carrier and spread evenly with the applicator tip of the fluoride bottle. Place carriers over the teeth and let it remain in place for one (1) minute or longer (as directed by your doctor). Expectorate the excess gel. Clean carriers with cold water. Spit out the excess gel in the mouth and do not rinse, eat, or drink for thirty (30) minutes.
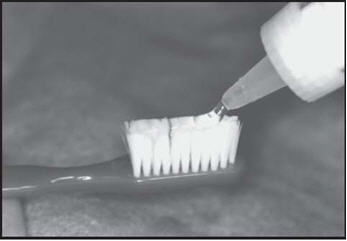
Brush On
Brush and floss your teeth to remove plaque. Adults and children 6 years of age or older: Apply a thin ribbon of gel to the toothbrush and spread evenly with the applicator tip of the fluoride bottle. Brush all tooth surfaces and allow fluoride to remain in place for one (1) minute or longer (as directed by your doctor). Clean carriers with cold water. Expectorate the excess gel in the mouth and do not rinse, eat, or drink for thirty (30) minutes.
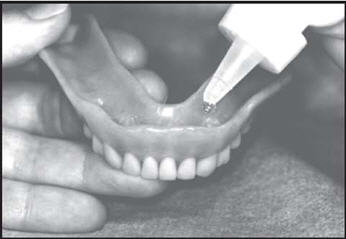
Overdentures
Brush all tooth surfaces. Clean your dentures. Adults and children 6 years of age or older: Apply one (1) or two (2)drops of FluoriShield to each tooth area in your denture and place the dentures in your mouth. Leave in place for one (1) minute or longer (as directed by your doctor).Expectorate the excess gel in the mouth and do not rinse, eat, or drink for thirty (30) minutes.