DR. RECKEWEG R40 DIAGLUKON COMBINATION PRODUCT- arsenicum album 8x, lycopodium clavatum 30x, natrum sulphuricum 12x, phosphoricum acidum 12x, secale cornutum 4x liquid
PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active ingredients
Arsenicum album 8X, Lycopodium clavatum 30X, Natrum sulphuricum 12X, Phosphoricum acidum 12X, Secale cornutum 4X, 1 g each in 10 g.
Purpose
Relieves symptoms associated with diabetes
Uses
For the temporary relief of minor symptoms associated with diabetes such as:
- Increased thirst
- Weakness
- Headaches
- Agitation
Warnings
Stop use and ask a doctor if symptoms persist, get worse, or if new symptoms occur.
If pregnant or breastfeeding, ask a health care professional before use.
Keep out of reach of children.
Directions
Adults and children ≥ 12 years 5-10 drops 1-3 times daily in a little water or undiluted or as recommended.
Other information
- Tamper resistant: do not use if safety ring at base of cap is broken.
- Retain outer carton for full product information.
Inactive ingredients
Ethanol, purified water.
Questions?
Call 1-800-361-7872 or email questions@reckeweg.com
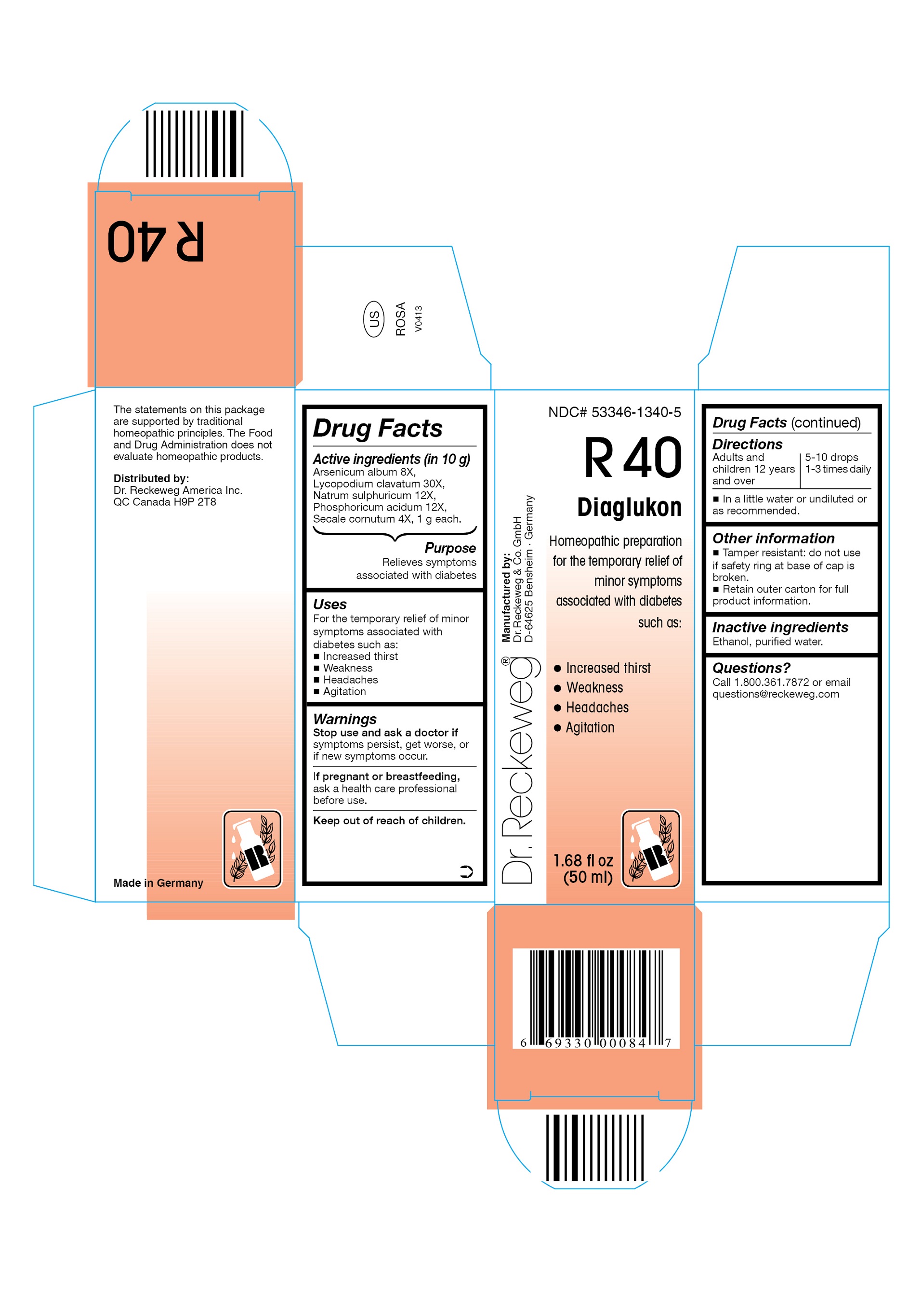
NDC# 53346-1340-5
Dr. Reckeweg R40 Diaglukon
Homeopathic preparation for the temporary relief of minor symptoms associated with diabetes such as:
- Increased thirst
- Weakness
- Headaches
- Agitation
Manufactured by:
Dr. Reckeweg Co. GmbH
D-64625 Bensheim
Germany
1.68 fl oz
(50 ml)
PHARMAZEUTISCHE FABRIK DR. RECKEWEG & CO
