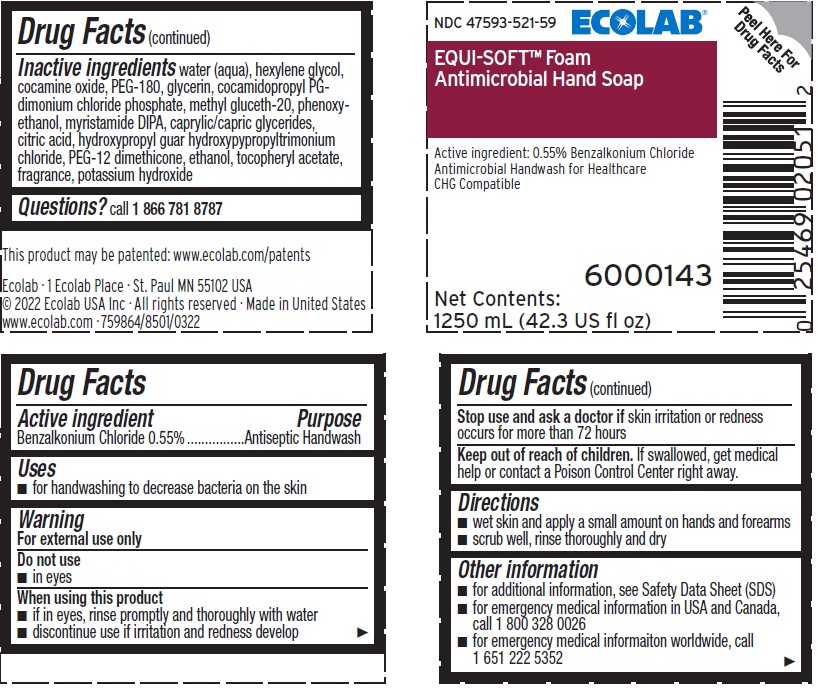
Warnings
- For external use only
Directions
- wet skin and spread a small amount on hands and forearms
- scrub well, rinse thoroughly and dry
Other information
- for additional information, see Safety Data Sheet (SDS)
- for emergency medical information in USA and Canada, call 1 800 328 0026
- for emergency medical information worldwide, call 1 651 222 5352
Inactive ingredients
water (aqua), hexylene glycol, cocamine oxide, PEG-180, glycerin, cocamidopropyl PG-dimonium chloride phosphate, methyl gluceth-20, phenoxyethanol, myristamide DIPA, caprylic/capric glycerides, citric acid, hydroxypropyl guar hydroxypypropyltrimonium chloride, PEG-12 dimethicone, ethanol, tocopheryl acetate, fragrance, potassium hydroxide
Representative Label and Principal Display Panel
47593-521-59
ECOLAB®
EQUI-SOFT™ Foam
ANTIMICROBIAL HAND SOAP
Active Ingredient: 0.55% Benzalkonium Chloride
Antimicrobial Handwash for Healthcare
CHG Compatible
6000143
1250 mL (42.3 US fl oz)
Ecolab · 1 Ecolab Place · St. Paul MN 55102 USA
© 2022 Ecolab USA Inc · All rights reserved · Made in United States
www.ecolab.com · 759864/8501/0322