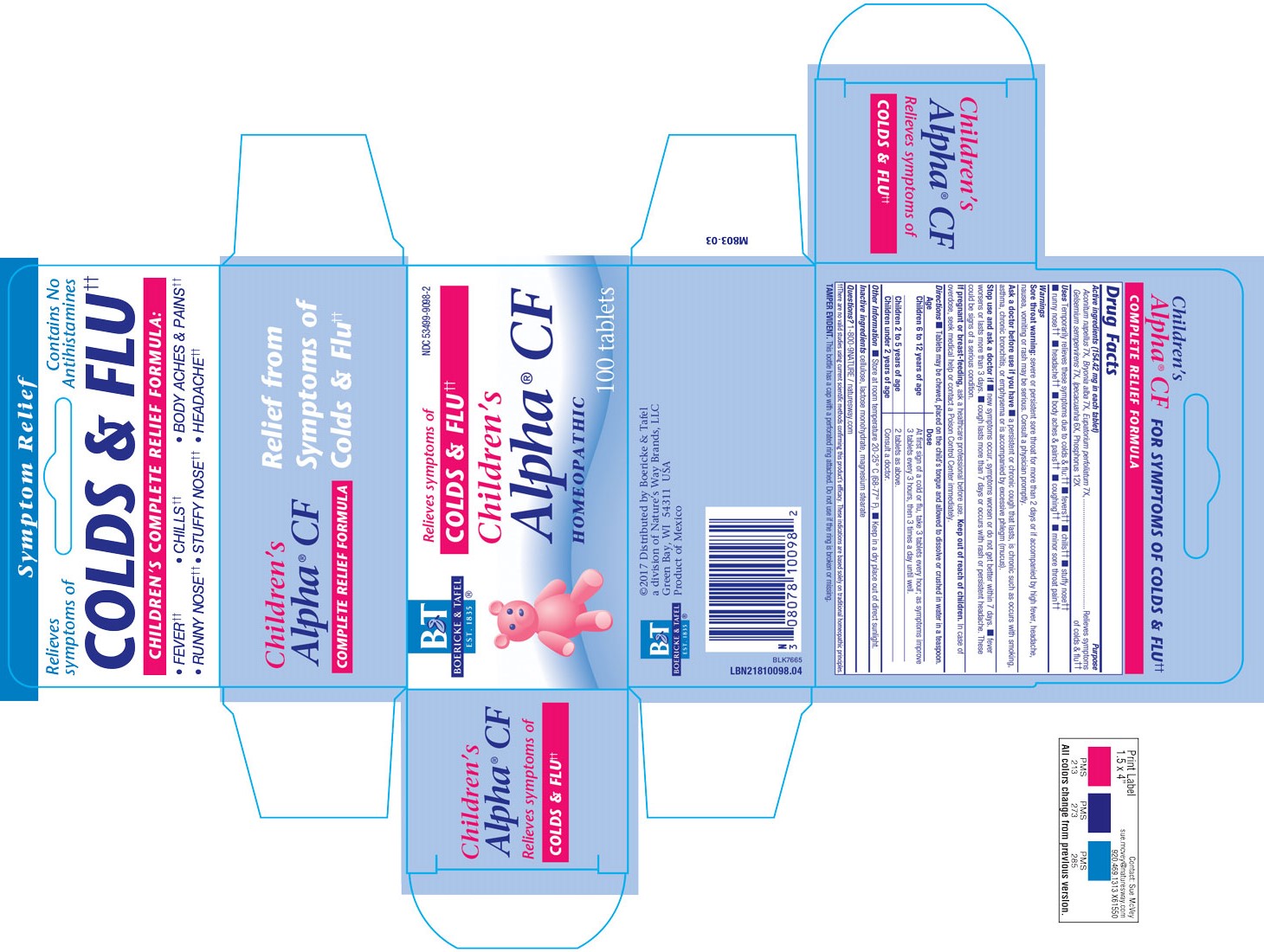
ACTIVE INGREDIENTS
Aconitum napellus 7X
Bryonia alba 7X
Eupatorium perfoliatum 7X
Gelsemium sempervirens 7X
Ipecacuanha 6X
Phosphorus 12X
Purpose
Temporarily relieves these symptoms due to colds and flu: fever, chills, stuffy nose, runny nose, headache, body aches and pains, coughing, minor sore throat pain.
Indications & Usage
Temporarily relieves these symptoms due to colds and flu: fever, chills, stuffy nose, runny nose, headache, body aches and pains, coughing, minor sore throat pain.
Dosage & Administration
Tablets may be chewed or placed on the child's tongue and allowed to dissolve or crushed in water in a teaspoon.
Children 6 to 12 years of age: At the first sign of cold or flu, take 3 tablets every hour; as symptoms improve 3 tablets every 3 hours, then 3 times a day until well.
Children under 2 to 5 years of age: 2 tablets as above.
Children under 2 years of age: Consult a doctor.
Warnings
Sore Throat Warning: Severe or persistent sore throat for more than 2 days or if accompanied by high fever, headache, nausea, vomiting, or rash may be serious.
Consult a physician promptly.
Ask Doctor
Ask a doctor before use if you have a persistent or chronic cough that lasts, is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema or is accomapnied by excessive phlegm (mucus).
Stop use
Stop use and ask a doctor if new symptoms occur, symptoms worsen or do not get better within 7 days, fever worsens or lasts more than 3 days, cough lasts more than 7 days or occurs with rash or persistent headache.
These could be signs of a serious condition.