Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6-12 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Directions
- take preferably at bedtime or as directed by a doctor
| Age | Starting Dosage | Maximum Dosage |
|---|---|---|
| adults and children 12 years of age or older | 2 softgels once a day | 4 softgels twice a day |
| children 6 to under 12 years | 1 softgel once a day | 2 softgels twice a day |
| children under 6 years of age | ask a doctor | ask a doctor |
Other information
- each softgel contains: calcium 1 mg and sodim less than 1 mg
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
Inactive ingredients
Aerosil, beeswax, FD&C Red No. 33, FD&C Yellow No.6, gelatin, glycerin, light liquid paraffin, purified water, sodium carboxymethyl cellulose, sorbitol solution, soya lecithin, titanium dioxide
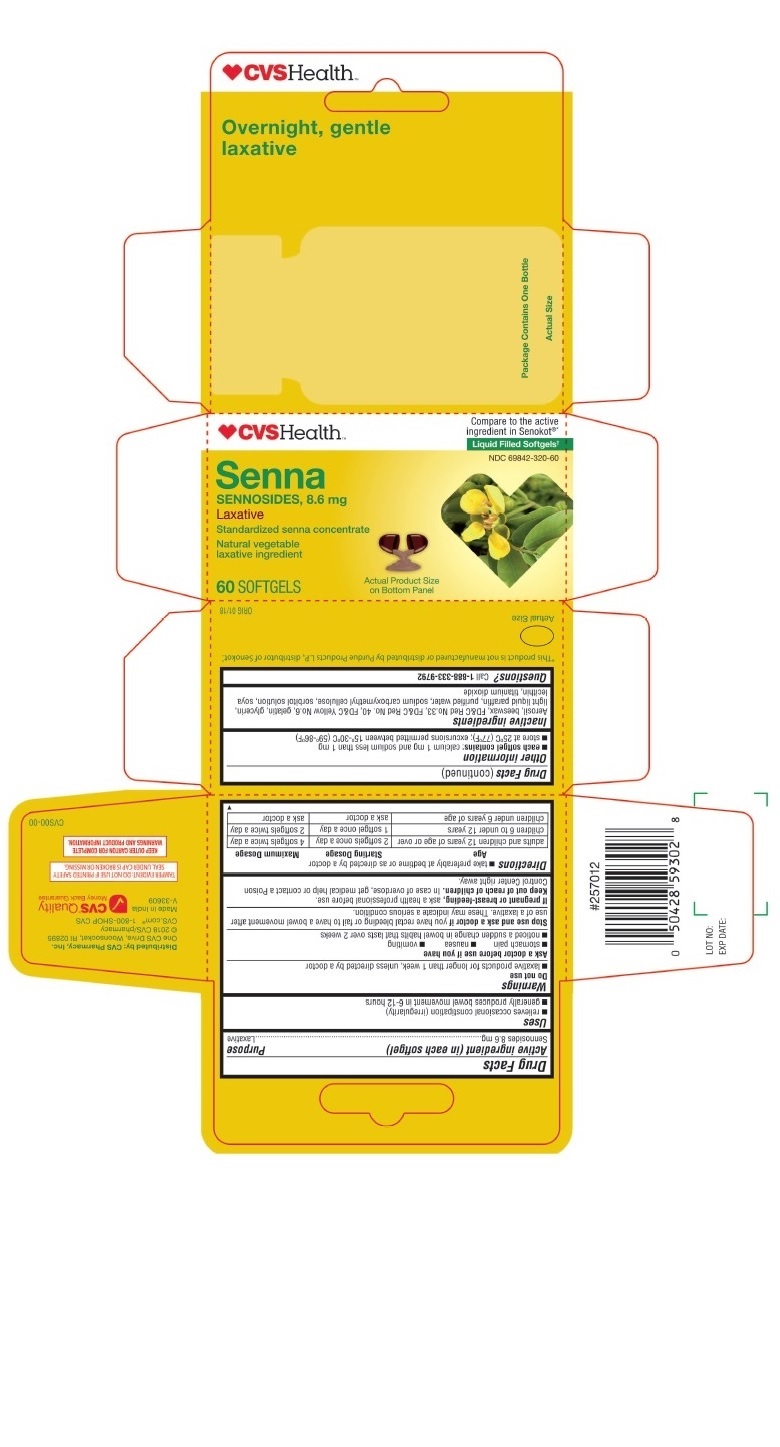
PRINCIPAL DISPLAY PANEL - CVS SENNA 60 Softgels Bottle Carton
♥CVSHealth ™
Compare to the active
ingredient in Senokot
®*
Liquid Filled Softgels†
NDC 69842-320-60
Senna
SENNOSIDES, 8.6 mg
Laxative
Standardized senna concentrate
Natural vegetable
laxative ingredient
60 SOFTGELS
Actual Product Size
on Bottom Panel