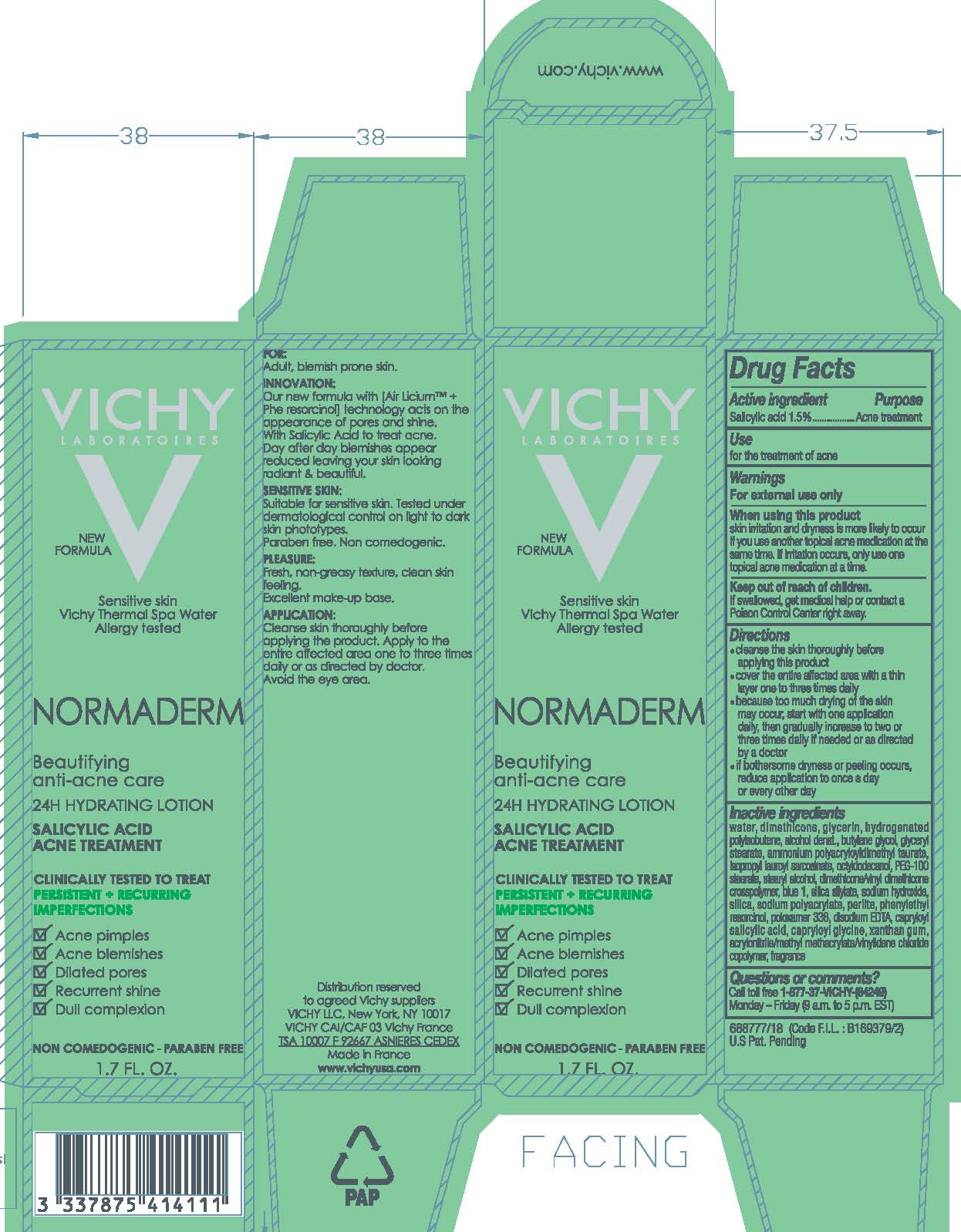
When using this product
skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- cleanse the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because too much drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
water, dimethicone, glycerin, hydrogenated polyisobutene, alcohol denat., butylene glycol, glyceryl stearate, ammonium polyacryloyldimethyl taurate, isopropyl lauroyl sarcosinate, octyldodecanol, PEG-100 stearate, stearyl alcohol, dimethicone/vinyl dimethicone crosspolymer blue 1, silica silylate, sodium hydroxide, silica, sodium polyacrylate, perlite, phenylethyl resorcinol, poloxamer 338, disodium EDTA, capryloyl salicylic acid, capryloyl glycine, xanthan gum, acrylonitrile/methyl methacrylate/vinylidene chloride copolymer, fragrance