HOMATROPINE HYDROBROMIDE
OPHTHALMIC SOLUTION, USP
Rx only
Established name:
Homatropine Hydrobromide
Chemical Name:
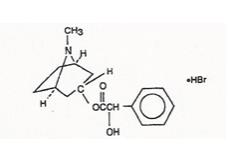
Benzeneacetic acid, ɑ-hydroxy-, 8-methyl-8-azabicyclo [3.2.1]-oct-3-yl ester, hydrobromide, endo-(±)-. The active ingredient is represented by the chemical structure:
Each mL contains: Active: Homatropine Hydrobromide 5.0%. Preservative: Benzalkonium Chloride 0.005%. Inactive: Boric Acid, Edetate Disodium, Potassium Chloride, Water for Injection. Boric Acid or Sodium Carbonate may be added to adjust the pH.
INDICATIONS AND USAGE:
A moderately long-acting mydriatic and cycloplegic for cycloplegic refraction and in the treatment of inflammatory conditions of the uveal tract. For pre and postoperative states when mydriasis is required. Use as an optical aid in some cases of axial lens opacities.
DOSAGE AND ADMINISTRATION:
For refraction, instill one or two drops topically in the eye(s). May be repeated in five or ten minutes if necessary. For uveitis, instill one or two drops topically up to every three to four hours. Individuals with heavily pigmented irides may require larger doses.
CONTRAINDICATIONS:
Contraindicated in persons with primary glaucoma or a tendency toward glaucoma, e.g. narrow anterior chamber angle, and in those persons showing hypersensitivity to any component of this preparation.
WARNING:
For topical use only – not for injection. Risk-benefit should be considered when the following medical problems exist: keratoconus (Homatropine may produce fixed dilated pupil); Down’s syndrome, children with brain damage and the elderly (increased susceptibility). In infants and small children, use with extreme caution. Excessive use in pediatric patients or certain individuals with a history of susceptibility to belladonna alkaloids may produce systemic symptoms of homatropine poisoning (see overdose section).
PRECAUTIONS:
General.
To avoid excessive systemic absorption, the lacrimal sac should be compressed by digital pressure for two to three minutes after installation. To avoid inducing angle closure glaucoma, an estimation of the depth of the angle of the anterior chamber should be made. Excessive topical use of this drug can potentially lead to a confusional state characterized by delirium, agitation, and rarely coma. This state is more apt to occur in the pediatric and geriatric age groups. The specific anti-dote for this systemic anticholinergic syndrome is injectable physostigmine salicylate.
Information to Patients.
Patient should be advised not to drive or engage in other hazardous activities while pupils are dilated. Patient may experience sensitivity to light and should protect eyes in bright illumination during dilation. Parents should be warned not to get this preparation in their child’s mouth and to wash their own hands and the child’s hands following administration. Do not touch dropper tip to any surface, as this may contaminate the solution.
ADVERSE REACTIONS:
Transient symptoms of stinging and burning may occur. Prolonged use may produce local irritation characterized by follicular conjunctivitis, vascular congestion, edema, exudates, and an eczematoid dermatitis. Thirst or dryness of mouth, eye irritation not present before therapy, or increased sensitivity of eyes to light may occur.
To report SUSPECTED ADVERSE REACTIONS, contact Altaire Pharmaceuticals, Inc. at (800)-258-2471.
Pregnancy.
Pregnancy Category C. Animal reproduction studies have not been conducted with homatropine hydrobromide. It is also not known whether homatropine hydrobromide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Homatropine Hydrobromide should be given to a pregnant woman only if clearly needed.
OVERDOSAGE:
When signs and symptoms of homatropine toxicity develop (see adverse reaction section), physostigmine should be administered parenterally (for dosage refer to Goodman & Gilman or other pharmacology reference). In infants and pediatric patients, the body surface must be kept moist.
DESCRIPTION:
Homatropine hydrobromide is an anticholinergic prepared as a sterile topical ophthalmic solution.
CLINICAL PHARMACOLOGY:
This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia).
