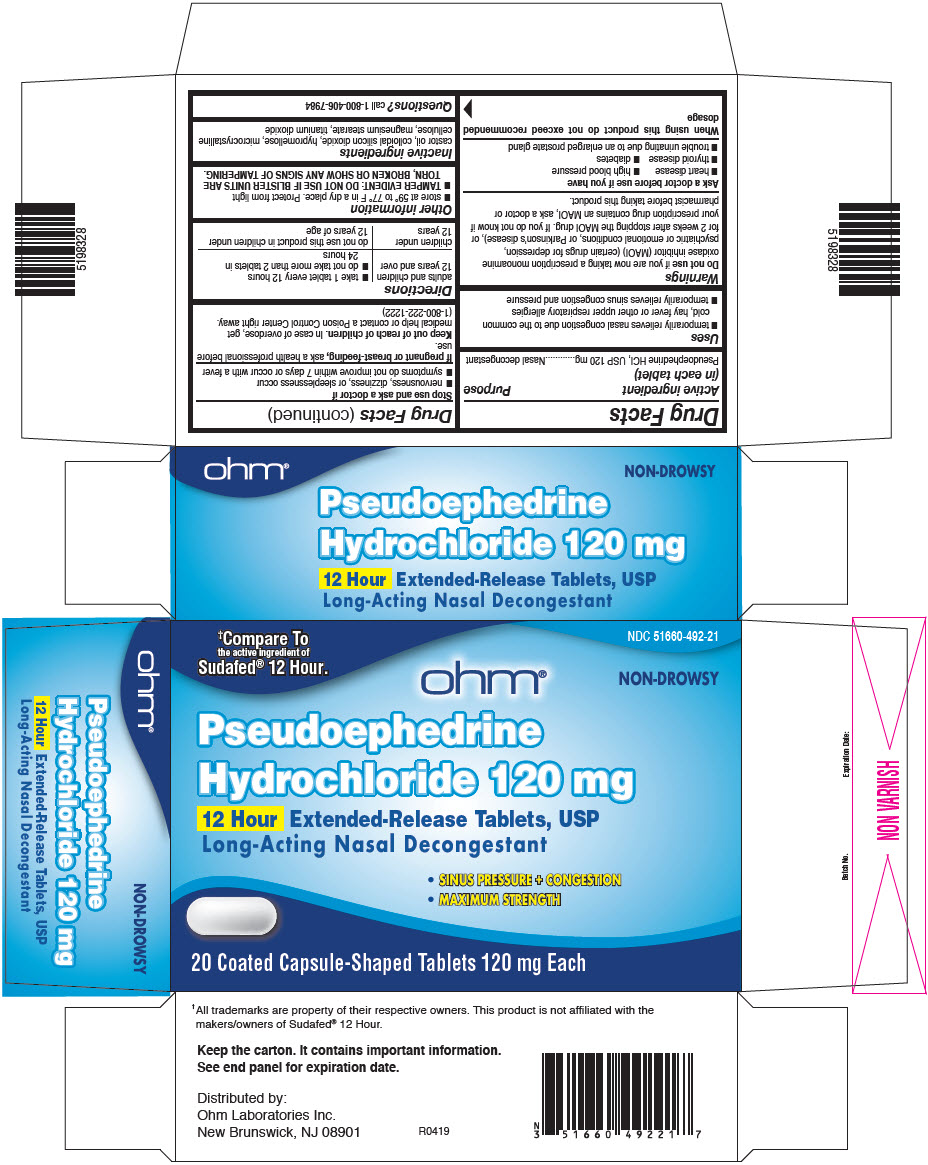
Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves sinus congestion and pressure
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
Directions
| adults and children 12 years and over |
|
| children under 12 years | do not use this product in children under 12 years of age |
Other information
- store at 59° to 77° F in a dry place. Protect from light
- TAMPER EVIDENT: DO NOT USE IF BLISTER UNITS ARE TORN, BROKEN OR SHOW ANY SIGNS OF TAMPERING.
Inactive ingredients
castor oil, colloidal silicon dioxide, hypromellose, microcrystalline cellulose, magnesium stearate, titanium dioxide
PRINCIPAL DISPLAY PANEL - 20 Tablet Blister Pack
†Compare To
the active ingredient of
Sudafed® 12 Hour.
NDC 51660-492-21
ohm®
NON-DROWSY
Pseudoephedrine
Hydrochloride 120 mg
12 Hour Extended-Release Tablets, USP
Long-Acting Nasal Decongestant
- SINUS PRESSURE + CONGESTION
- MAXIMUM STRENGTH
20 Coated Capsule-Shaped Tablets 120 mg Each