Uses
- for the temporary relief of the following cold/flu symptoms:
- minor aches and pains
- headache
- sore throat
- nasal congestion
- cough
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
- temporarily reduces fever
Warnings
Liver warning
This product contains acetaminophen. The maximum daily dose of this product is 10 caplets (3,250 mg acetaminophen) in 24 hours. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion or cough gets worse or lasts more than 7 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back or occurs with rash or headache that lasts
These could be signs of a serious condition.
Directions
- do not take more than directed (see overdose warning)
| adults and children 12 years and over |
|
| children under 12 years | ask a doctor |
Other information
- each caplet contains: sodium 3 mg
- store between 20-25°C (68-77°F)
- do not use if pouch is torn or damaged
Inactive ingredients
carnauba wax, croscarmellose sodium, D&C yellow no. 10 aluminum lake, flavor, hydroxypropyl cellulose, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch, sucralose, titanium dioxide
Product distributed by: JOHNSON & JOHNSON CONSUMER INC.
McNeil Consumer Healthcare Division, Fort Washington, PA 19034 USA
Repackaged and distributed by: Lil' Drug Store Products, Inc., 9300 Earhart Lane SW
Cedar Rapids, IA 52402
PRINCIPAL DISPLAY PANEL - 6 Caplet Pouch Carton
TYLENOL®
FOR ADULTS
COLD + FLU SEVERE
Acetaminophen, Dextromethorphan HBr, Phenylephrine HCl, Guaifenesin
Pain Reliever–Fever Reducer, Cough Suppressant, Nasal Decongestant, Expectorant
- HEAD + BODY ACHES
- FEVER + SORE THROAT
- COUGH
- NASAL CONGESTION
- MUCUS + CHEST CONGESTION
6
Caplets
3 POUCHES OF 2 CAPLETS EACH
Lil'
Drug Store
®

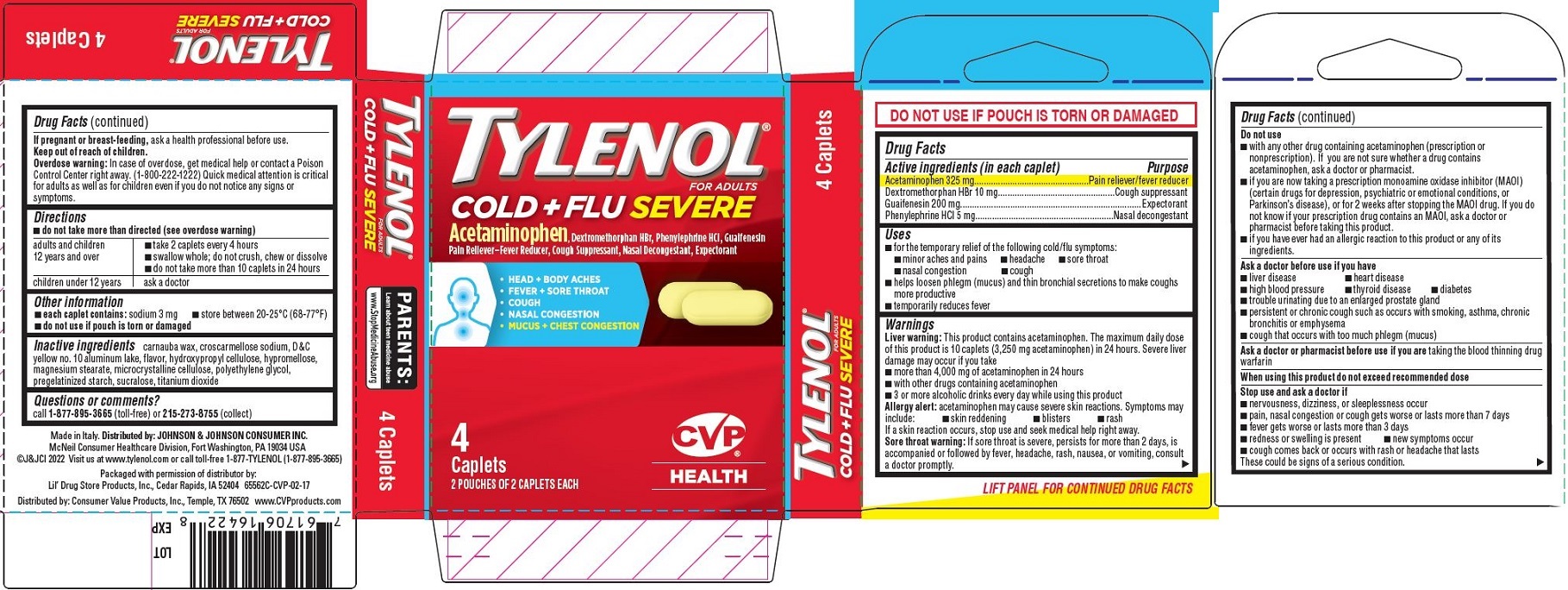
Tylenol Cold + Flu SEVERE, CVP ® Health 4ct - PDP/Package
TYLENOL
®
FOR ADULTS
COLD + FLU SEVERE
Acetaminophen, Dextromethorphan HBr, Phenylephrine HCl, Guaifenesin
Pain Reliever–Fever Reducer, Cough Suppressant, Nasal Decongestant, Expectorant
HEAD + BODY ACHES
FEVER + SORE THROAT
COUGH
NASAL CONGESTION
MUCUS + CHEST CONGESTION
[caplets image]
4
Caplets
2 POUCHES OF 2 CAPLETS EACH
[CVP HEALTH logo]
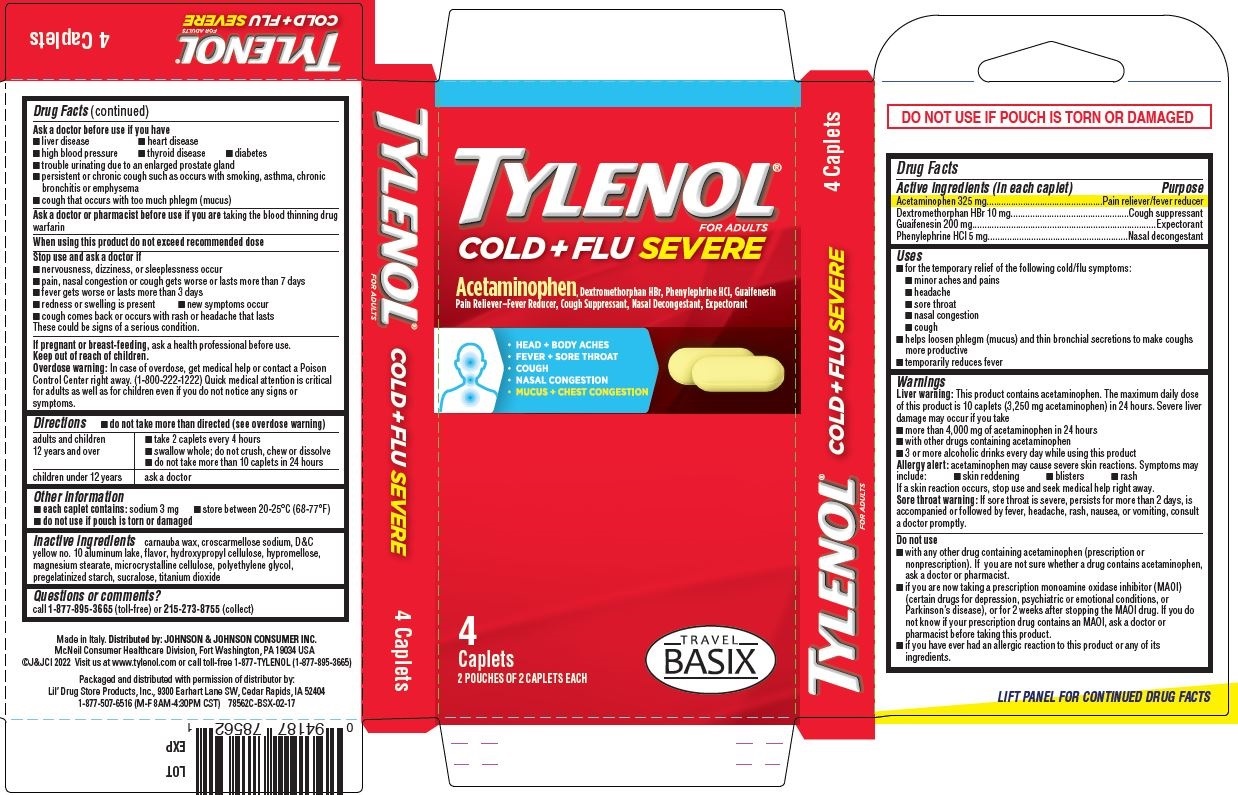
Tylenol Cold + Flu SEVERE, TRAVEL BASIX 4ct - PDP/Package
TYLENOL®
FOR ADULTS
COLD + FLU SEVERE
Acetaminophen, Dextromethorphan HBr, Phenylephrine HCl, Guaifenesin
Pain Reliever–Fever Reducer, Cough Suppressant, Nasal Decongestant, Expectorant
HEAD + BODY ACHES
FEVER + SORE THROAT
COUGH
NASAL CONGESTION
MUCUS + CHEST CONGESTION
[caplets image]
4
Caplets
2 POUCHES OF 2 CAPLETS EACH
[TRAVEL BASIX logo]