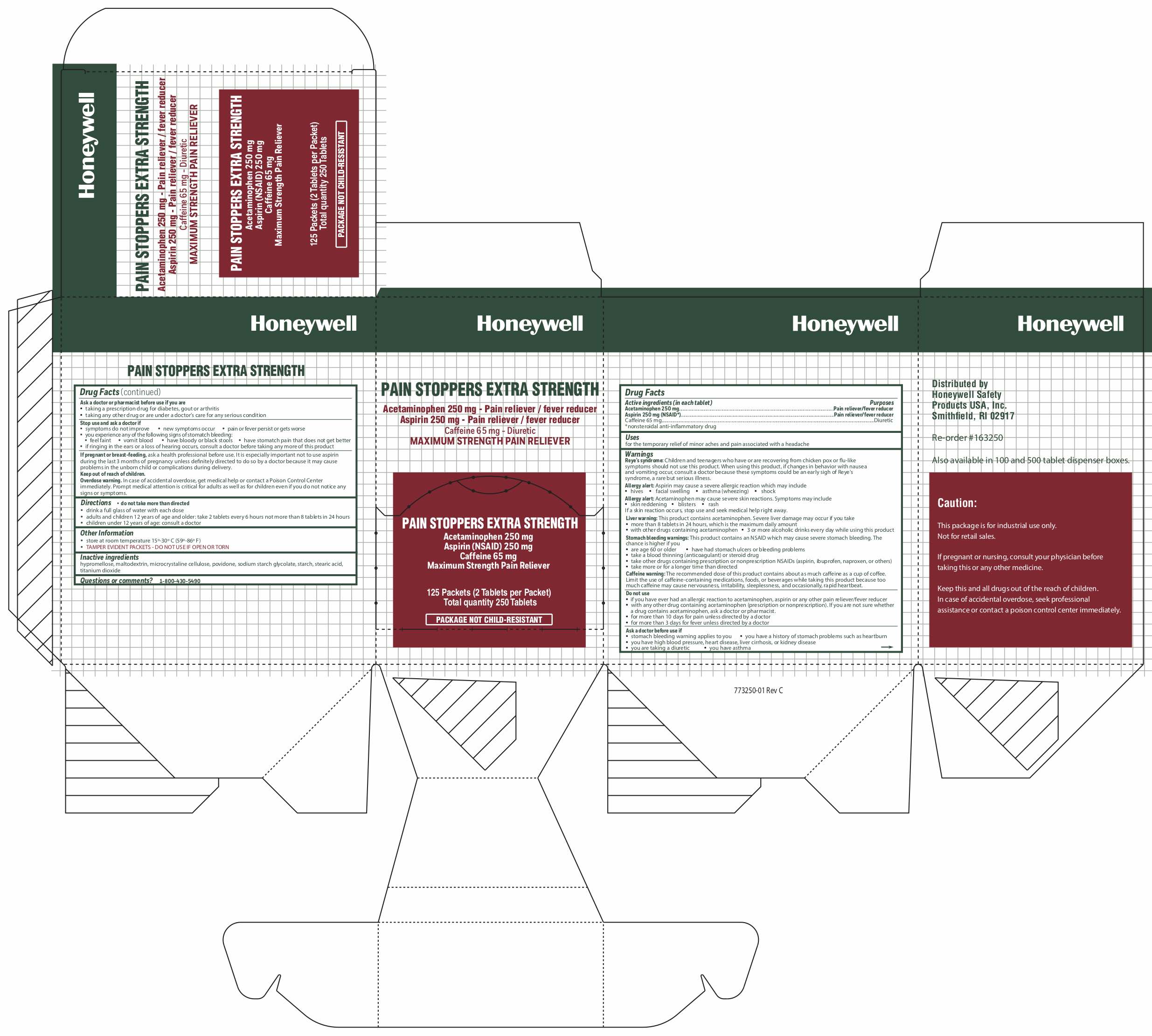
PAIN STOPPERS EX- acetaminophen, caffeine, aspirin tablet, coated
Honeywell Safety Products USA, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-2521: Pain Stoppers EX
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sigh of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock.
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 8 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Stomach Bleeding Warning: This product contains an NSAID, which may cause stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing NSAIDs (aspirin, ibuprofen, naproxen, or others).
- Take more or for a longer time than directed
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, food or beverages, while taking this product because too much caffeine may cause nervousness, sleeplessness, irritability, occasionally, rapid heartbeat
Do not use
- if you have ever had an allergic reaction to acetaminophen, aspirin or any other pain reliever/fever reducer
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- for more than 10 days for pain unless directed by a doctor
- for more than 3 days for fever unless directed by a doctor
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you are taking a diuretic
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you have asthma
Ask a doctor or pharmacist before use if you are
- taking a prescription drug for diabetes, gout or arthritis
- taking any other drug or are under a doctor's care for any serious condition
Stop use and ask a doctor if
- symptoms do not improve
- new symptoms occur
- pain or fever persists or gets worse
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- if ringing in the ears or a loss of hearing occurs, consult a doctor before taking any more of this product.
Directions
- do not take more than directed
- drink a full glass of water with each dose
- adults and children 12 years of age and older: take 2 tablets every 6 hours not more than 8 tablets in 24 hours
- children under 12 yearsof age: consult a doctor
Other Information
- store at room temperature 15 o-30 o C (59 o-86 o F)
- TAMPER EVIDENT - DO NOT USE IF OPEN OR TORN
| PAIN STOPPERS EX
acetaminophen, caffeine, aspirin tablet, coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc (118768815) |