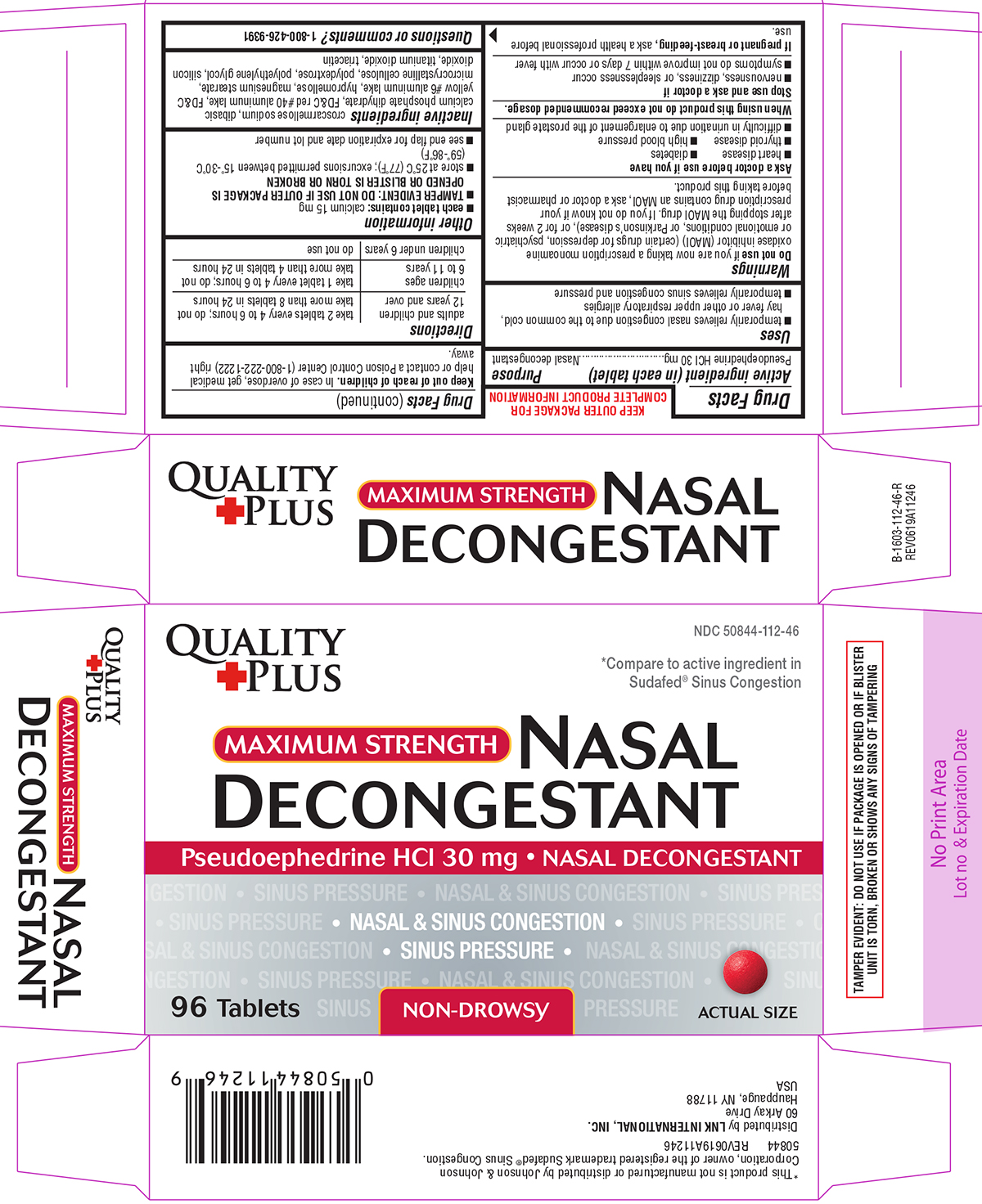
Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves sinus congestion and pressure
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- diabetes
- heart disease
- high blood pressure
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
Directions
|
adults and children | take 2 tablets every 4 to 6 hours; do not take more than 8 tablets in 24 hours |
| children ages 6 to 11 years | take 1 tablet every 4 to 6 hours; do not take more than 4 tablets in 24 hours |
|
children under 6 years |
do not use |
Other information
- each tablet contains: calcium 15 mg
-
TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, silicon dioxide, titanium dioxide, triacetin
Principal Display Panel
QUALITY
PLUS
NDC 50844-112-46
*Compare to active ingredient in
Sudafed® Sinus Congestion
MAXIMUM STRENGTH
NASAL
DECONGESTANT
Pseudoephedrine HCl 30 mg • NASAL DECONGESTANT
• NASAL & SINUS CONGESTION •
• SINUS PRESSURE •
96 Tablets
NON-DROWSY
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Johnson & Johnson
Corporation, owner of the registered trademark Sudafed® Sinus Congestion.
50844 REV0619A11246
Distributed by LNK INTERNATIONAL, INC.
60 Arkay Drive,
Hauppauge, NY 11788
USA
Quality Plus 44-112