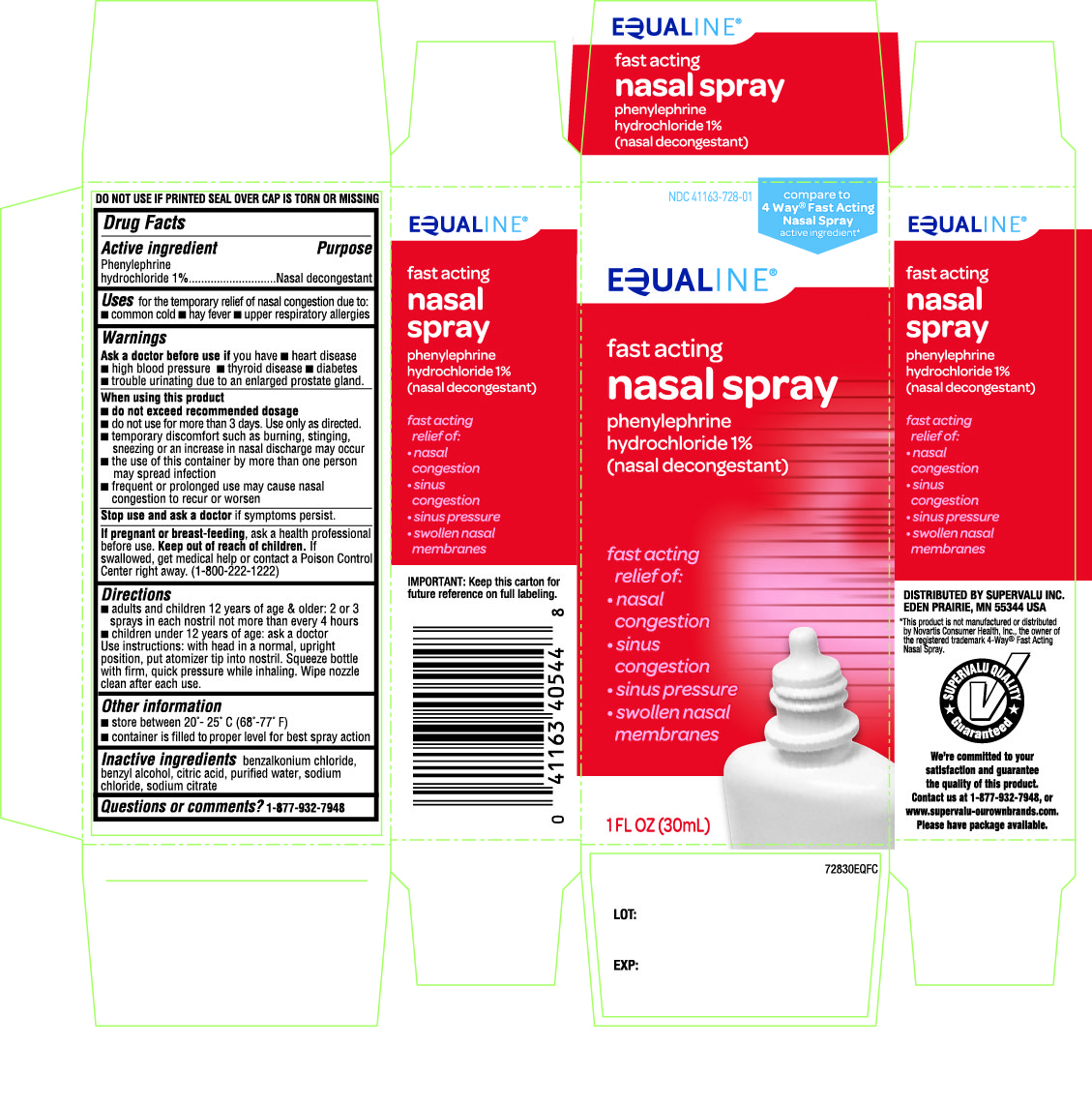
Uses
for the temporary relief of nasal congestion due to:
- •
- common cold
- •
- hay fever
- •
- upper respiratory allergies
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
When using this product
• do not exceed recommended dosage
• do not use for more than 3 days. Use only as directed
• temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge may occur
• the use of this container by more than one person may spread infection
• frequent or prolonged use may cause nasal congestion to recur or worsen
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away (1-800-222-1222)
Directions
- •
- adults and children 12 years of age and over: 2 or 3 sprays in each nostril not more than every 4 hours
- •
- children under 12 years of age: ask a doctor
- •
- Use instructions: with head in a normal, upright position, put atomizer tip into nostril. Squeeze bottle with firm, quick pressure while inhaling. Wipe nozzle clean after each use.
Other information
- •
- store between 20°-25°C (68°-77°F)
- •
- container is filled to proper level for best spray action
Inactive Ingredients
benzalkonium chloride, benzyl alcohol, citric acid, purified water, sodium chloride, sodium citrate
Questions
1-877-932-7948
IMPORTANT: Keep this carton for future reference on full labeling
DO NOT USE IF PRINTED SEAL OVER CAP IS TORN OR MISSING
Distributed By: Supervalu Inc.
Eden Prairie, MN 55344
This product is not manufactured or distributed by Novartis Consumer Health Inc., the owner of the registered trademark 4-Way Fast Acting Nasal Spray.