CESTEX- epsiprantel tablet, film coated
Pfizer Animal Health
----------
Cestex® (epsiprantel) Veterinary Tablets
CAUTION
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Cestex (epsiprantel) tablets are indicated for the removal of tapeworms in the cat (Dipylidium caninum and Taenia taeniaeformis) and dog (Dipylidium caninum and Taenia pisiformis).
DESCRIPTION
Cestex tablets are film-coated and contain 12.5 mg, 25 mg, 50 mg or 100 mg of epsiprantel per tablet.
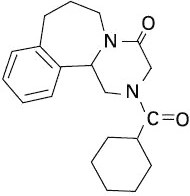
Epsiprantel is an anthelmintic that is active as a single dose against the common tapeworms of cats and dogs. Epsiprantel has a molecular weight of 326 and is chemically 2-(cyclohexyl-carbonyl)-4-oxo 1,2,3,4,6,7,8,12b-octahydropyrazino[2,1-a][2]benza-zepine. It is a stable white solid which is sparingly soluble in water. Its chemical structure is presented below.
ACTION
Epsiprantel acts directly on the tapeworm. Since it is minimally absorbed following oral administration, epsiprantel remains at the site of action within the gastrointestinal tract. Due to digestive process, tapeworm fragments or proglottids may not be readily visible in the stool.
INDICATIONS
Cestex tablets are indicated for the removal of the following:
Feline cestodes: Dipylidium caninum and Taenia taeniaeformis
Canine cestodes: Dipylidium caninum and Taenia pisiformis
DOSAGE AND ADMINISTRATION
Cestex tablets should be administered orally.
The recommended dosage of epsiprantel is: cats, 1.25 mg/lb of body weight; dogs, 2.5 mg/lb of body weight. The following table may be used as a guide:
| Feline | |
| Body Weight | Dose |
| Seven weeks old up to 10 lb | 12.5 mg |
| 11–20 lb | 25.0 mg |
| Canine | |
| Body Weight | Dose |
| Seven weeks old up to 5 lb | 12.5 mg |
| 6–10 lb | 25.0 mg |
| 11–20 lb | 50.0 mg |
| 21–40 lb | 100.0 mg |
| 41–50 lb | 125.0 mg |
| 51–60 lb | 150.0 mg |
| 61–80 lb | 200.0 mg |
| 81–90 lb | 225.0 mg |
| 91–100 lb | 250.0 mg |
| 101+ lb | 2.5 mg/lb, rounding up to next whole tablet combination |
Fasting is not necessary or recommended.
Unless exposure to the infected intermediate hosts is controlled, reinfection is likely and retreatment may be required. In the case of D. caninum, an effective flea control program should be instituted.
SAFETY
Epsiprantel has been evaluated in cats at 5 times the recommended dose given once daily for 3 days with no adverse effects noted. In tolerance studies, epsiprantel produced minimal clinical signs in cats given 40 times the recommended dose once daily for 4 days.
Epsiprantel has been evaluated in 14-day repeat dose studies in dogs at 500 mg/kg (90 times recommended dosage) with no significant adverse results. No side effects were observed during the clinical field studies.
Epsiprantel is not a cholinesterase inhibitor. During the course of clinical field studies, Cestex was administered concurrently with diethylcarbamazine citrate (dogs only), anti-inflammatory agents, insecticides, and nematocides with no drug incompatibilities noted.
WARNINGS
For use in cats and dogs only. Safety of use in pregnant or breeding animals has not been determined. Keep out of reach of children. Not for use in humans.
HOW SUPPLIED
Cestex tablets are film-coated and contain 12.5 mg, 25 mg, 50 mg or 100 mg of epsiprantel per tablet. Cestex is supplied as described below:
| Weight (lbs) | Number Tablets/Bottle | ||
|---|---|---|---|
| Concentration | Cat | Dog | |
| 12.5 mg | 10 | 5 | 50, 100 |
| 25.0 mg | 20 | 10 | 50, 100 |
| 50.0 mg | – – | 20 | 25, 50 |
| 100.0 mg | – – | 40 | 25, 50 |
PRINCIPAL DISPLAY PANEL - 50 12.5 mg Tablet Bottle Label
Cestex®
(epsiprantel)
Caution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.
12.5 mg
50 tablets
NADA #140-893, Approved by FDA
Pfizer

PRINCIPAL DISPLAY PANEL - 100 12.5 Tablet Bottle Label
Cestex®
(epsiprantel)
Caution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.
12.5 mg
100 tablets
NADA #140-893, Approved by FDA
Pfizer

PRINCIPAL DISPLAY PANEL - 50 25 mg Tablet Bottle Label
Cestex®
(epsiprantel)
Caution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.
25 mg
50 tablets
NADA #140-893, Approved by FDA
Pfizer

PRINCIPAL DISPLAY PANEL - 100 25 mg Tablet Bottle Label
Cestex®
(epsiprantel)
Caution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.
25 mg
100 tablets
NADA #140-893, Approved by FDA
Pfizer

PRINCIPAL DISPLAY PANEL - 25 50 mg Tablet Bottle Label
Cestex®
(epsiprantel)
Caution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.
50 mg
25 tablets
NADA #140-893, Approved by FDA
Pfizer

PRINCIPAL DISPLAY PANEL - 50 50 mg Tablet Bottle Label
Cestex®
(epsiprantel)
Caution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.
50 mg
50 tablets
NADA #140-893, Approved by FDA
Pfizer

| CESTEX
epsiprantel tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CESTEX
epsiprantel tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CESTEX
epsiprantel tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| CESTEX
epsiprantel tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pfizer Animal Health (039055157) |
| Registrant - Pfizer Inc. (943955690) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Animal Health | 039055157 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Emcure Pharmaceuticals Ltd., India, Pune | 862602830 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| UQUIFA MEXICO S.A. DE. C.V. | 810105874 | API MANUFACTURE | |


