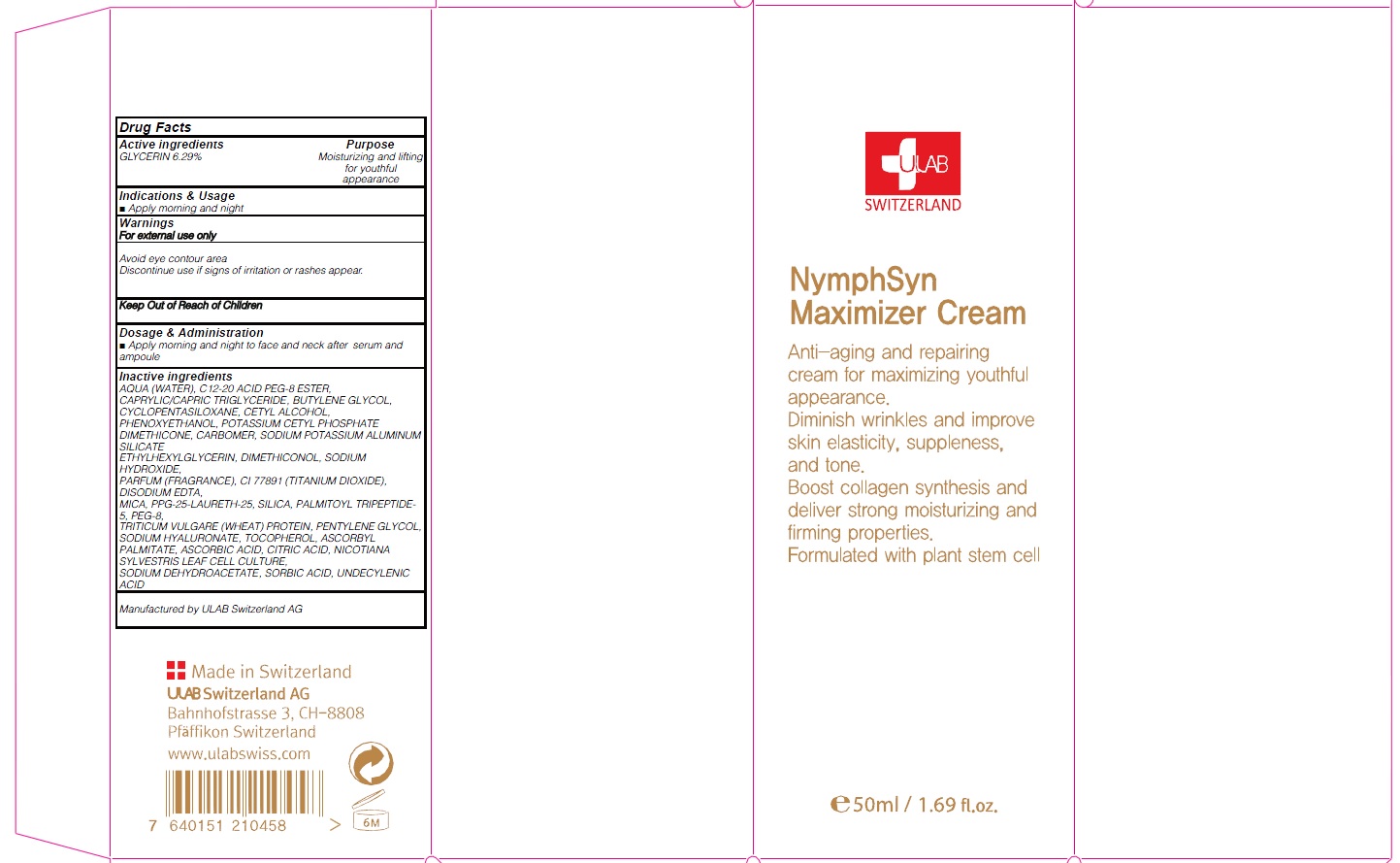
INACTIVE INGREDIENT
Inactive ingredients: AQUA (WATER), C12-20 ACID PEG-8 ESTER, CAPRYLIC/CAPRIC TRIGLYCERIDE, BUTYLENE GLYCOL, CYCLOPENTASILOXANE, CETYL ALCOHOL, PHENOXYETHANOL, POTASSIUM CETYL PHOSPHATE DIMETHICONE, CARBOMER, SODIUM POTASSIUM ALUMINUM SILICATE ETHYLHEXYLGLYCERIN, DIMETHICONOL, SODIUM HYDROXIDE, PARFUM (FRAGRANCE), CI 77891 (TITANIUM DIOXIDE), DISODIUM EDTA, MICA, PPG-25-LAURETH-25, SILICA, PALMITOYL TRIPEPTIDE-5, PEG-8, TRITICUM VULGARE (WHEAT) PROTEIN, PENTYLENE GLYCOL, SODIUM HYALURONATE, TOCOPHEROL, ASCORBYL PALMITATE, ASCORBIC ACID, CITRIC ACID, NICOTIANA SYLVESTRIS LEAF CELL CULTURE, SODIUM DEHYDROACETATE, SORBIC ACID, UNDECYLENIC ACID
WARNINGS
Warnings: For external use only Avoid eye contour area Discontinue use if signs of irritation or rashes appear.