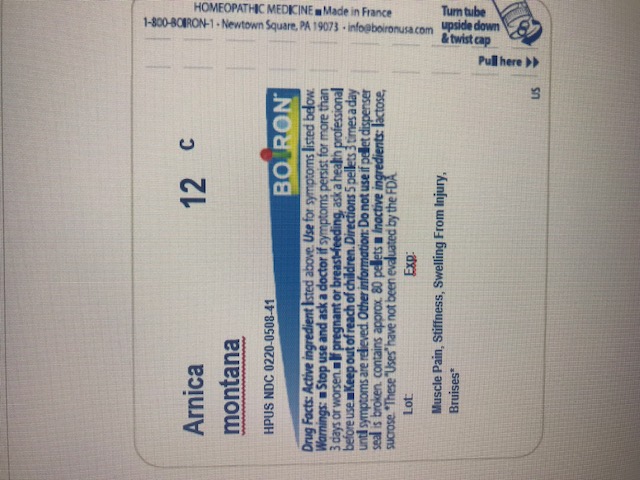
Active ingredient** (in each pellet)
Arnica montana 12C (0.443 mg)
The letters "HPUS" indicate that the component in this product is officially monographed in the Homeopathic Pharmacopoeia of the United States.
Uses*
temporarily relieves muscle pain and stiffness due to:
- minor injuries
- overexertion
- falls
- minor procedures
reduces symptoms of bruising such as:
- pain
- swelling
- discoloration
Stop use and ask a doctor if symptoms persist for more than 3 days or get worse, new symptoms occur, or redness or swelling is present. These could be signs of a serious condition.
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away.
Directions
Before procedure: Dissolve 5 pellets under the tongue 3 times a day for 2 days.
After procedure: Dissolve 5 pellets under the tongue 3 times a day until symptoms are relieved.
Questions, Comments?
www.boironusa.com
info@boironusa.com
1-800-BOIRON-1
(1-800-264-7661)
Newtown Square, PA 19073-3267
do not use if pellet dispenser seal is broken
3 tubes of Boiron Arnica montana 12C** HPUS
Provides approximately 15 days of treatment
Made in France
Boiron Arnica montana pellets reduce bruising, swelling, and pain used before and after your procedure.
Non-habit forming
Non-drowsy
No known drug interactions
post-operative care*
Dissolve 5 pellets under the tongue 3 times a day for 2 days.
Dissolve 5 pellets under the tongue 3 times a day until symptoms are relieved.
Turn tube upside down. Twist until 5 pellets are dispensed. Remove cap and pour pellets under the tongue.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
*C, K, CK and X are homeopathic dilutions: see BoironUSA.com/info for details.