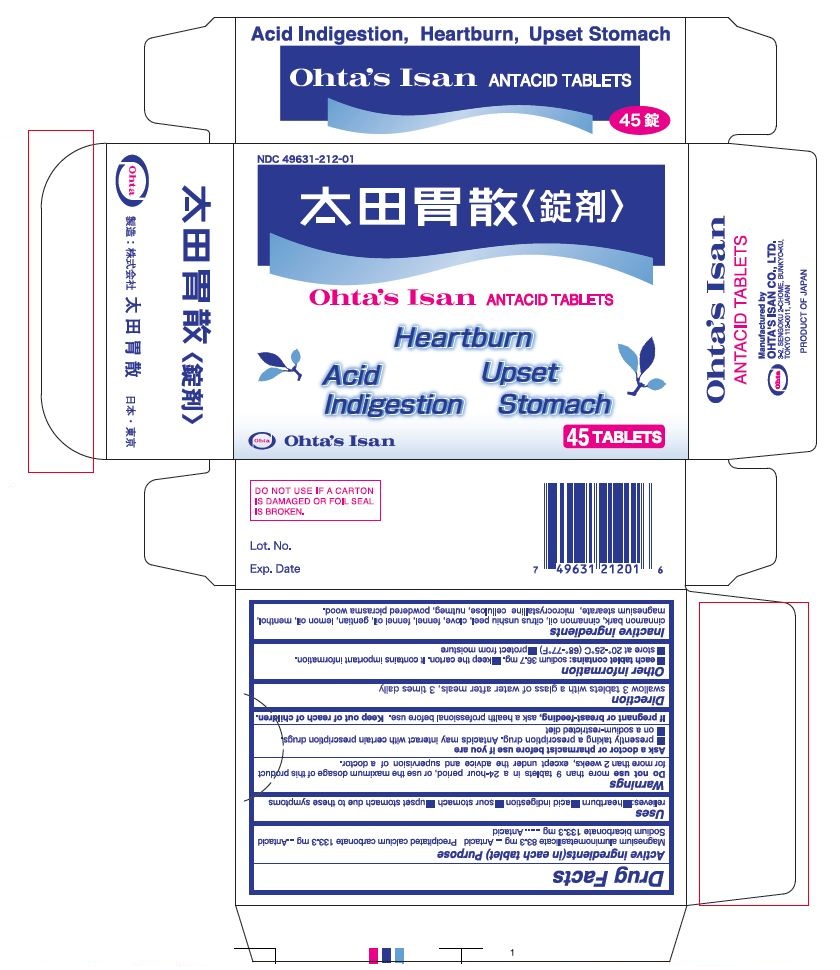
Active Ingredients (in each tablet)
Magnesium aluminometasilicate 83.3 mg
Precipitated calcium carbonate 133.3 mg
Sodium bicarbonate 133.3 mg
Purpose
Magnesium aluminometasilicate ............ Antacid
Precipitated calcium carbonate ............. Antacid
Sodium bicarbonate ............................. Antacid
Warnings
Do not use more than 9 tablets in a 24-hour period, or use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a doctor.
Ask a doctor or pharmacist before use if you are
■ presently taking a prescription drug. Antacids may interact with certain prescription drugs
■ on a sodium-restricted diet
Other Information
■ each tablet contains: sodium 36.7 mg. ■ keep the carton. It contains important information.
■ store at 20°-25°C (68°-77°F) ■ protect from moisture
Inactive ingredients
cinnamon bark, cinnamon oil, citrus unshiu peel, clove, fennel, fennel oil, gentian, lemon oil, menthol, magnesium stearate, microcrystalline cellulose, nutmeg, powdered picrasma wood.
PRINCIPAL DISPLAY PANEL
NDC 49631-212-01
Ohta's Isan ANTACID TABLETS
Heartburn Acid Indigestion Upset Stomach
45 TABLETS
Manufactured by
OHTA'S ISAN CO., LTD.
3-2, SENGOKU 2-CHOME, BUNKYO-KU,
TOKYO 112-0011, JAPAN
PRODUCT OF JAPAN
DO NOT USE IF A CARTON IS DAMAGED OR FOIL SEAL IS BROKEN.
Lot. No.
Exp. Date