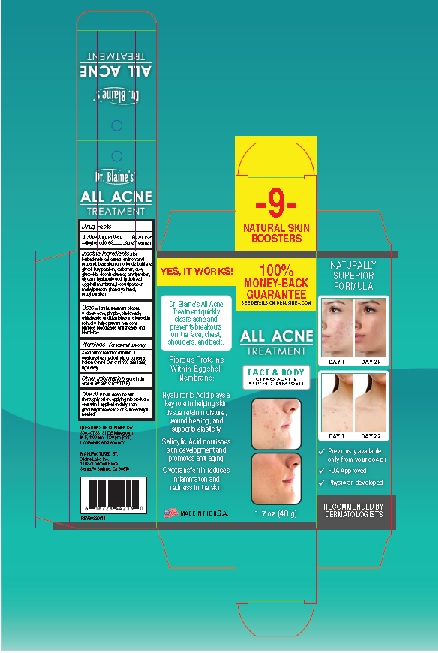
Active Ingredient Salycilic Acid 0.5%
Uses ■ for the treatment of acne
■ clears acne pimples, blackheads,
whiteheads and blemishes ■ allows skin
to heal ■ helps prevent new acne
pimples, blackheads whiteheads, and
acne blemishec
Warnings - For external use only
Keep out of reach of children. If
swallowed get medical help or contact a
Poison Control Center (1-800-222-1222)
right away.
Other Information store in dry
area at 20º-25º C (68º-77º F)
Directions ■ clean the skin
thoroughly before applying this product
■ start with1 application daily, then
gradually increase to 2 to 3 times daily if
needed
QUESTIONS OR COMMENTS?
800.307.8818 | DrBlaines.com
M-F, 8:00 am - 4:00 pm (PST)
Contact@BlaineLabs.com
Inactive Ingredients Aloe
barbadensis leaf extract, aminomethyl
propanol, benzalkonium chloride, butylene
glycol, butylparaben, carbomer, decyl
glucoside, deionized water, ethylparaben,
glycerin, hyaluronic acid, hydrolyzed
eggshell membrane, isobutylparaben,
methylparaben, phenoxyethanol,
propylparaben
Product Label
Dr. Blaine's®
ALL ACNE
TREATMENT
FACE & BODY
CLEARS ACNE &
PREVENTS BREAKOUTS
1.7 OZ (48 g)
MANUFACTURED BY:
Blaine Labs, Inc.
11037 Lockport Place
Santa Fe Springs, CA 90670

res
Blaine Labs Inc.

