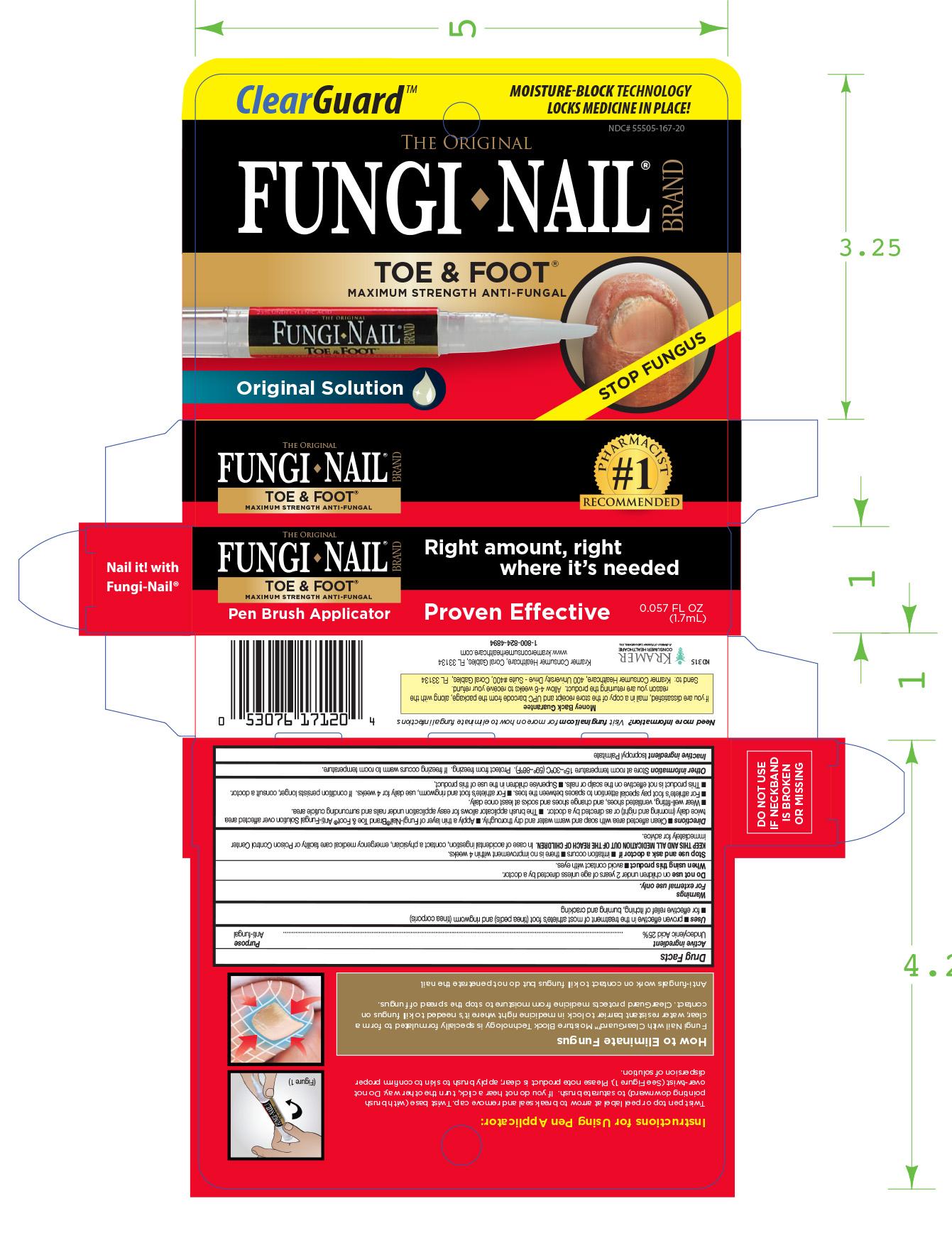
FUNGI NAIL TOE AND FOOT PEN- undecylenic acid liquid
Kramer Laboratories
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. In case of accidental ingestion, contact a physician, emergency medical care facility or Poison Control Center
immediately for advice.
Uses ■ proven effective in the treatment of most athlete’s foot (tinea pedis) and ringworm (tinea corporis)
■ for effective relief of itching, bur ning and cracking.
Directions ■ Clean affected are a with soap and warm water and dry thoroughly. ■ Apply a thin layer of Fungi-Nail®Brand Toe & Foot™ Anti-Fungal Solution over affected area
twice daily (morning and night) or as directed by a doctor. ■ The brush applicator allows for easy application under nails and surrounding cuticle area.
| FUNGI NAIL TOE AND FOOT PEN
undecylenic acid liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Kramer Laboratories (122720675) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Denison Pharmaceuticals, LLC | 001207208 | manufacture(55505-167) | |