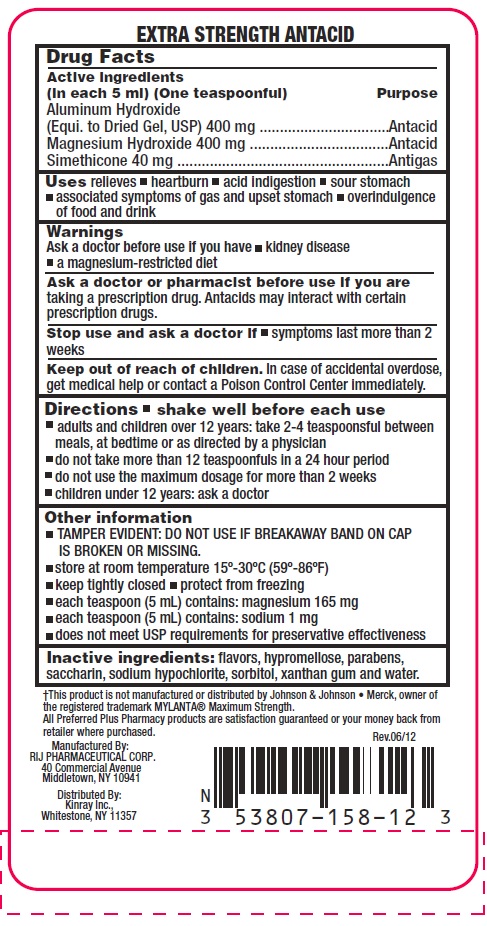
Active ingredients (in each 5 mL) (One teaspoonful)
Aluminum Hydroxide (Equi. to Dried Gel, USP) 400 mg
Magnesium Hydroxide 400 mg
Simethicone 40 mg
Uses
relieves
- •
- heartburn
- •
- acid indigestion
- •
- sour stomach
- •
- associated symptoms of gas and upset stomach
- •
- overindulgence of food and drink
Warnings
Directions
- •
- shake well before each use
- •
- adults and children 12 years, take 2 - 4 teaspoonsful between meals, at bedtime or as directed by a physician
- •
- do not take more than 12 teaspoonsful in 24 hour period
- •
- do not use the maximum dosage for more than 2 weeks
- •
- children under 12 years: ask a doctor
Other information
- •
- TAMPER EVIDENT: DO NOT USE IF BREAKAWAY BAND ON CAP IS BROKEN OR MISSING.
- •
- Store at room temperature 15º - 30ºC (59º - 86ºF)
- •
- keep tightly closed
- •
- protect from freezing
- •
- each teaspoon (5 mL) contains: magnesium 165 mg
- •
- each teaspoon (5 mL) contains: sodium 1 mg
- •
- does not meet USP requirements for preservative effectiveness