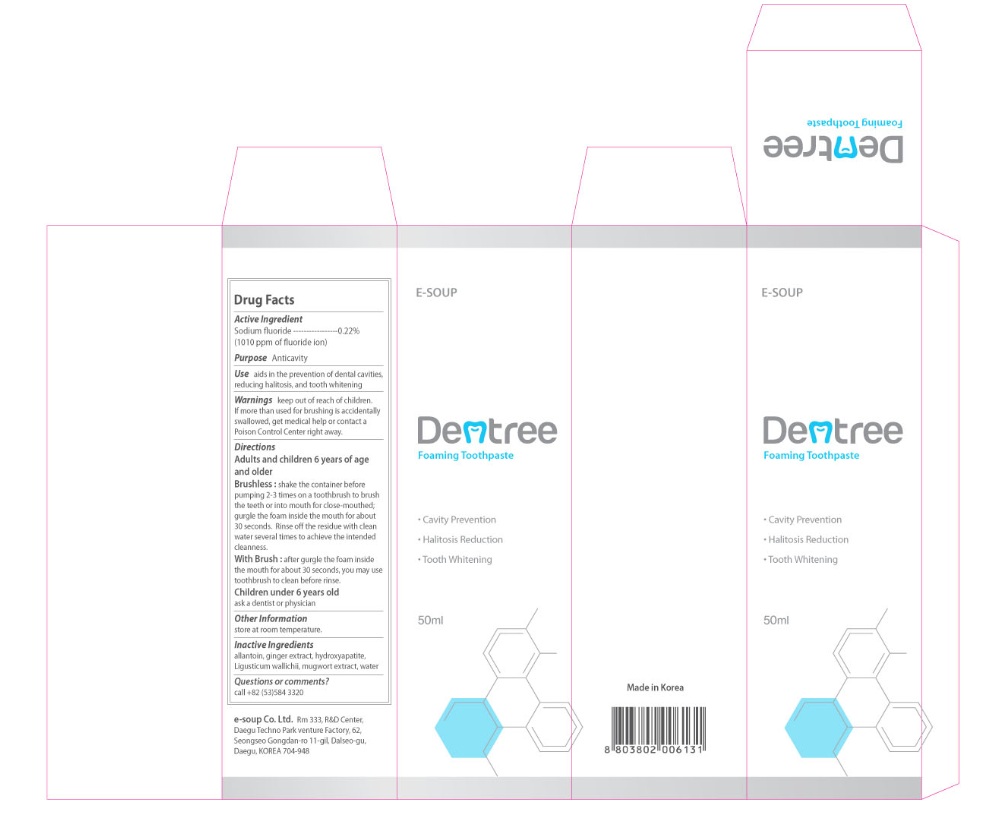
Warnings
keep out of reach of children.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 6 years of age and older
Brushless: shake the container before pumping 2-3 times on a toothbrush to brush the teeth or into mouth for close-mouthed, gurgle the foam inside the mouth for about 30 seconds. Rinse off the residue with clean water several times to achieve the intended cleanliness.
With Brush: after gurgle the foam inside the mouth for about 30 seconds, you may use toothbrush to clean before rinse.
Children under 6 years old
ask a dentist or physician