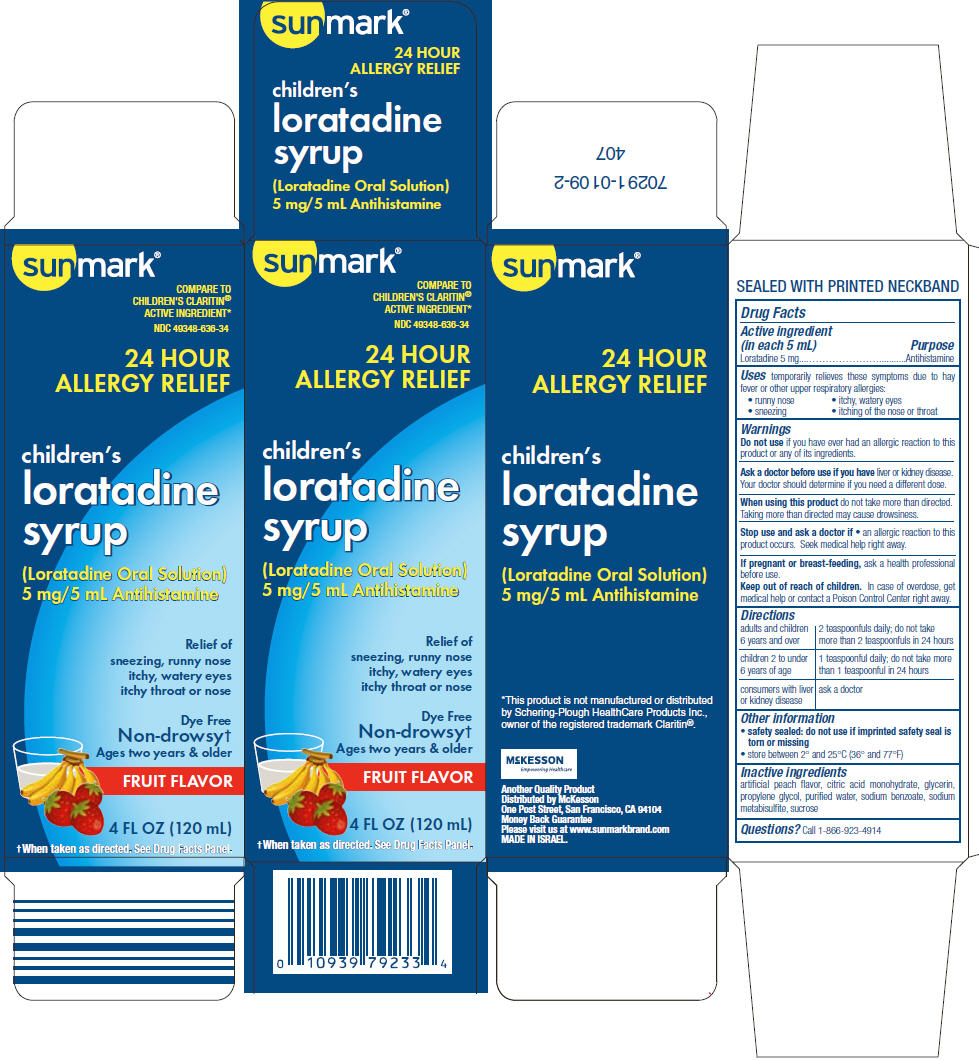
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
Directions
| adults and children 6 years and over | 2 teaspoonfuls daily; do not take more than 2 teaspoonfuls in 24 hours |
| children 2 to under 6 years of age | 1 teaspoonful daily; do not take more than 1 teaspoonful in 24 hours |
| consumers with liver or kidney disease | ask a doctor |
Other information
- safety sealed: do not use if imprinted safety seal is torn or missing
- store between 2° and 25°C (36° and 77°F)
Inactive ingredients
artificial peach flavor, citric acid monohydrate, glycerin, propylene glycol, purified water, sodium benzoate, sodium metabisulfite, sucrose
PRINCIPAL DISPLAY PANEL - 120 mL Bottle Carton
sunmark®
COMPARE TO
CHILDREN'S CLARITIN®
ACTIVE INGREDIENT*
NDC 49348-636-34
24 HOUR
ALLERGY RELIEF
children's
loratadine
syrup
(Loratadine Oral Solution)
5 mg/5 mL Antihistamine
Relief of
sneezing, runny nose
itchy, watery eyes
itchy throat or nose
Dye Free
Non-drowsy†
Ages two years & older
FRUIT FLAVOR
4 FL OZ (120 mL)
†When taken as directed. See Drug Facts Panel.