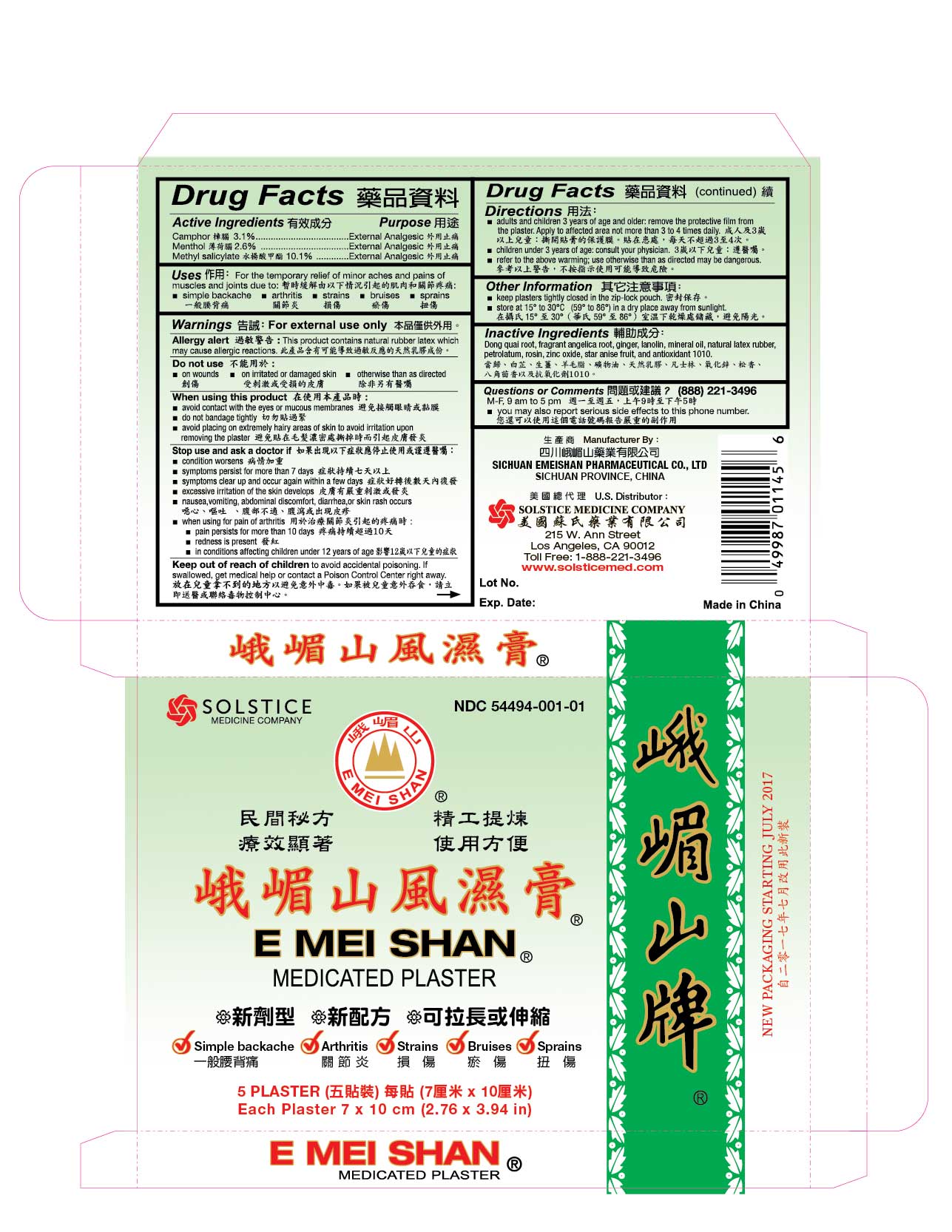
Uses
For the temporary relief of minor aches and pains of muscles and joints due to:
■ simple backache ■ arthritis ■ strains ■ bruises ■ sprains
Warnings
For external use only
Allergy alert: This product contains natural rubber latex which may cause allergic reactions
When using this product
■ avoid contact with the eyes or mucous membranes
■ do not bandage tightly
■ avoid placing on extremely hairy areas of skin to avoid irritation upon removing the plaster
Stop use and ask a doctor if
■ condition worsens
■ symptoms persist for more than 7 days
■ symptoms clear up and occur again within a few days
■ excessive irritation of the skin develops
■ nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
■ when using for pain of arthritis:
■ pain persists for more than 10 days ■ redness is present
■ in conditions affecting children under 12 years of age
Keep out of reach of children to avoid accidental poisoning.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
■ adults and children 3 years of age and older: remove the protective film from the plaster. Apply to affected area not more than 3 to 4 times daily.
■ children under 3 years of age: consult your physician
■ refer to the above warnings; use otherwise than as directed may be dangerous
Other information
■ keep plasters tightly closed in the zip-lock pouch
■ store at 15º to 30º C (59º to 86º F) in a dry place away from sunlight
Inactive ingredients
Dong quai root, fragrant angelica root, ginger, lanolin, mineral oil, natural latex rubber, petrolatum, rosin, zinc oxide, star anise fruit, and antioxidant 1010.