Uses
- temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
- sinus congestion and pressure
- nasal congestion
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- high blood pressure
- heart disease
- thyroid disease
- diabetes
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
When using this product
- do not exceed recommended dosage
- excitability may occur, especially in children
- drowsiness may occur
- avoid alcoholic beverages
- alcohol, sedatives and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
Directions
- adults and children 12 years and over: take 1 tablet every 4 hours. Do not take more than 6 tablets in 24 hours.
- children under 12 years: do not use
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, lactose anhydrous, magnesium stearate, microcrystalline cellulose, silicon dioxide, stearic acid
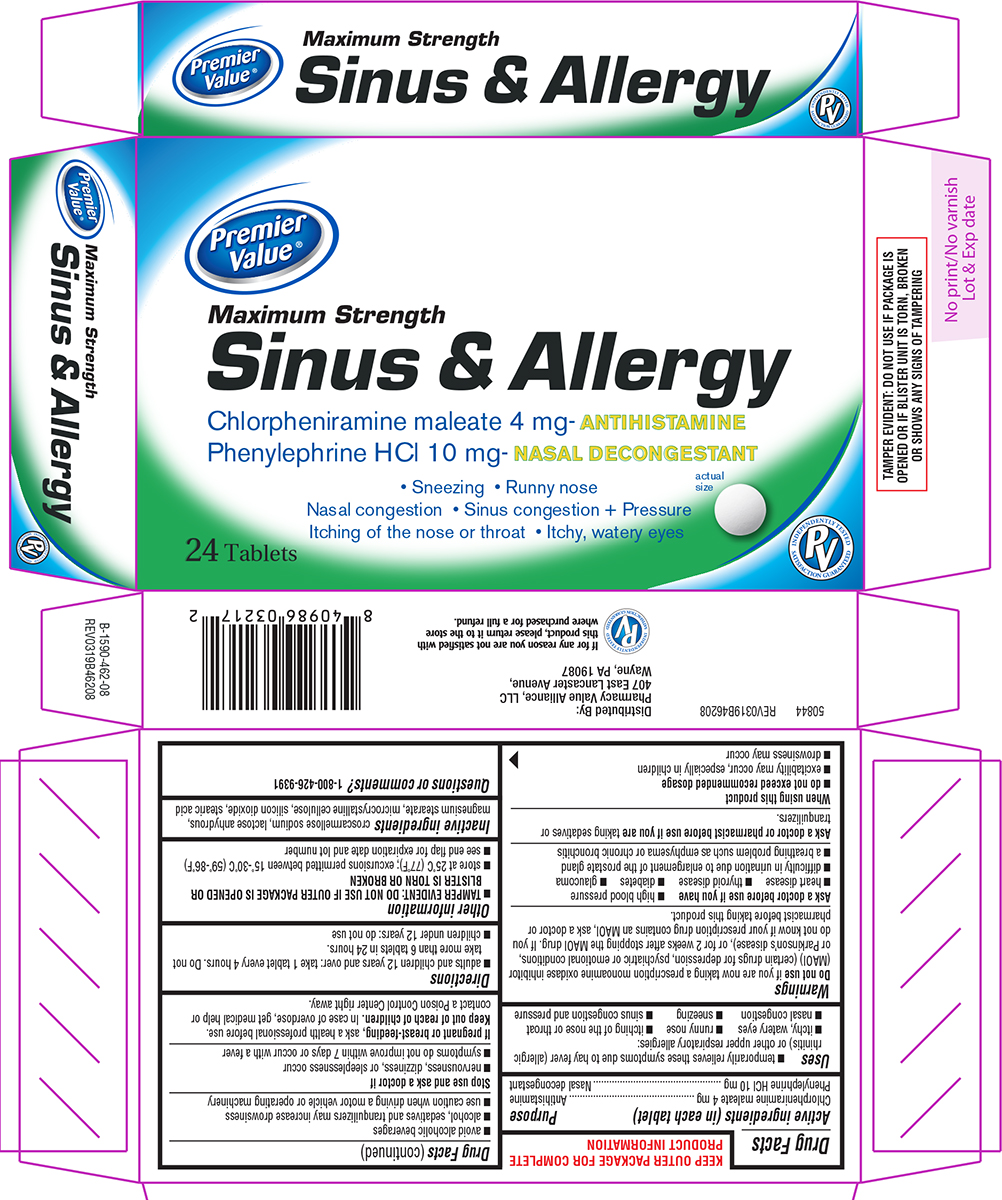
Principal Display Panel
Premier
Value®
Maximum Strength
Sinus & Allergy
Chlorpheniramine maleate 4 mg - ANTIHISTAMINE
Phenylephrine HCl 10 mg - NASAL DECONGESTANT
• Sneezing • Runny nose
Nasal congestion • Sinus congestion + Pressure
Itching of the nose or throat • Itchy, watery eyes
24 Tablets
actual size
INDEPENDENTLY TESTED
PV
SATISFACTION GUARANTEED
TAMPER EVIDENT: DO NOT USE IF CARTON IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERING
50844 REV0319B46208
Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087
If for any reason you are not satisfied with
this product, please return it to the store
where purchased for a full refund.
Premier Value 44-462