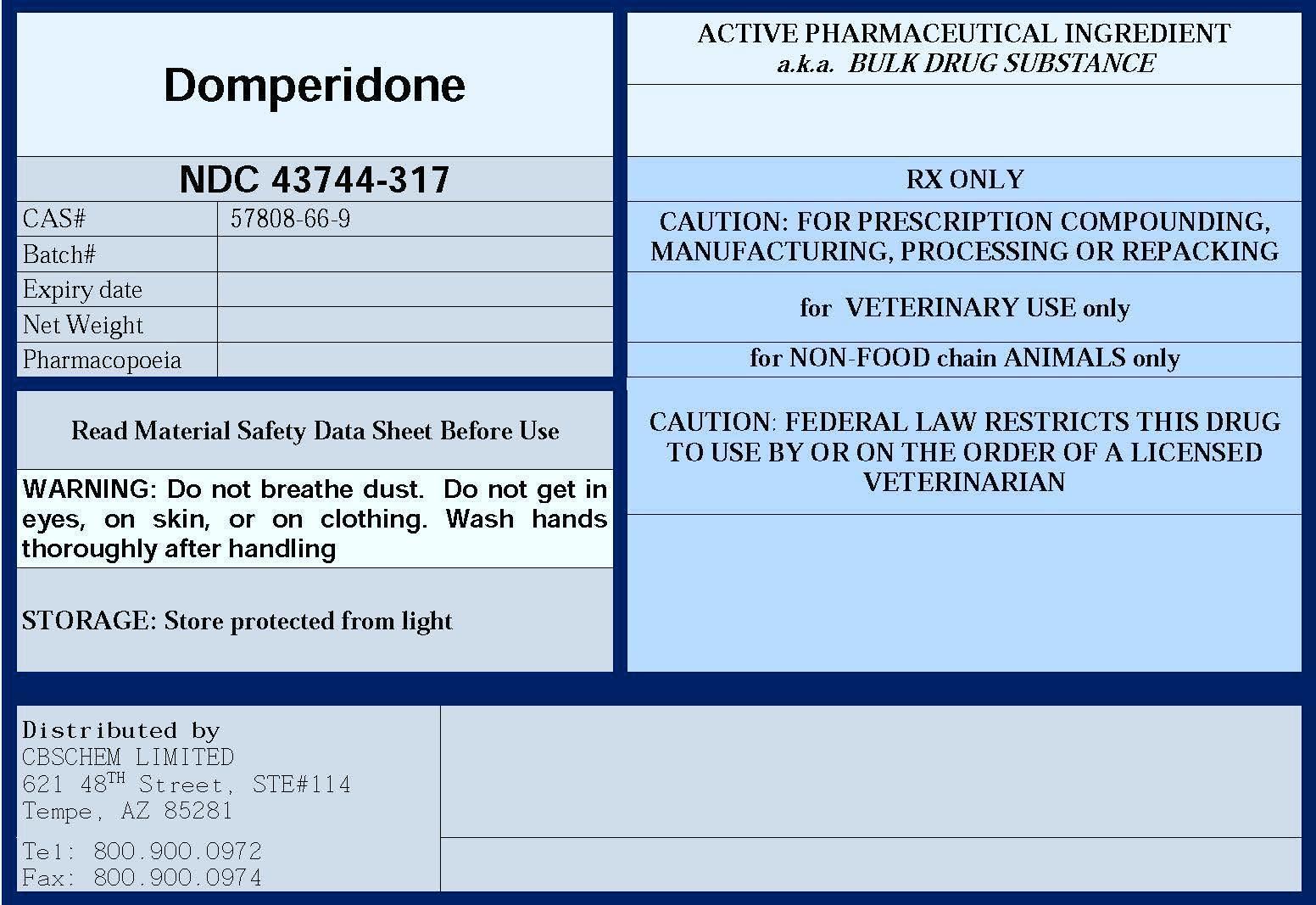
DOMPERIDONE- domperidone powder
CBSCHEM LIMITED
----------
DOMPERIDONE
| DOMPERIDONE
domperidone powder |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - CBSCHEM LIMITED (831801258) |
Revised: 6/2017
Document Id: a4ac0bdb-6a85-4513-8a22-4426a43d053d
Set id: ec55df7c-dfc3-4fb8-8286-2359ad1ddd62
Version: 8
Effective Time: 20170628
CBSCHEM LIMITED