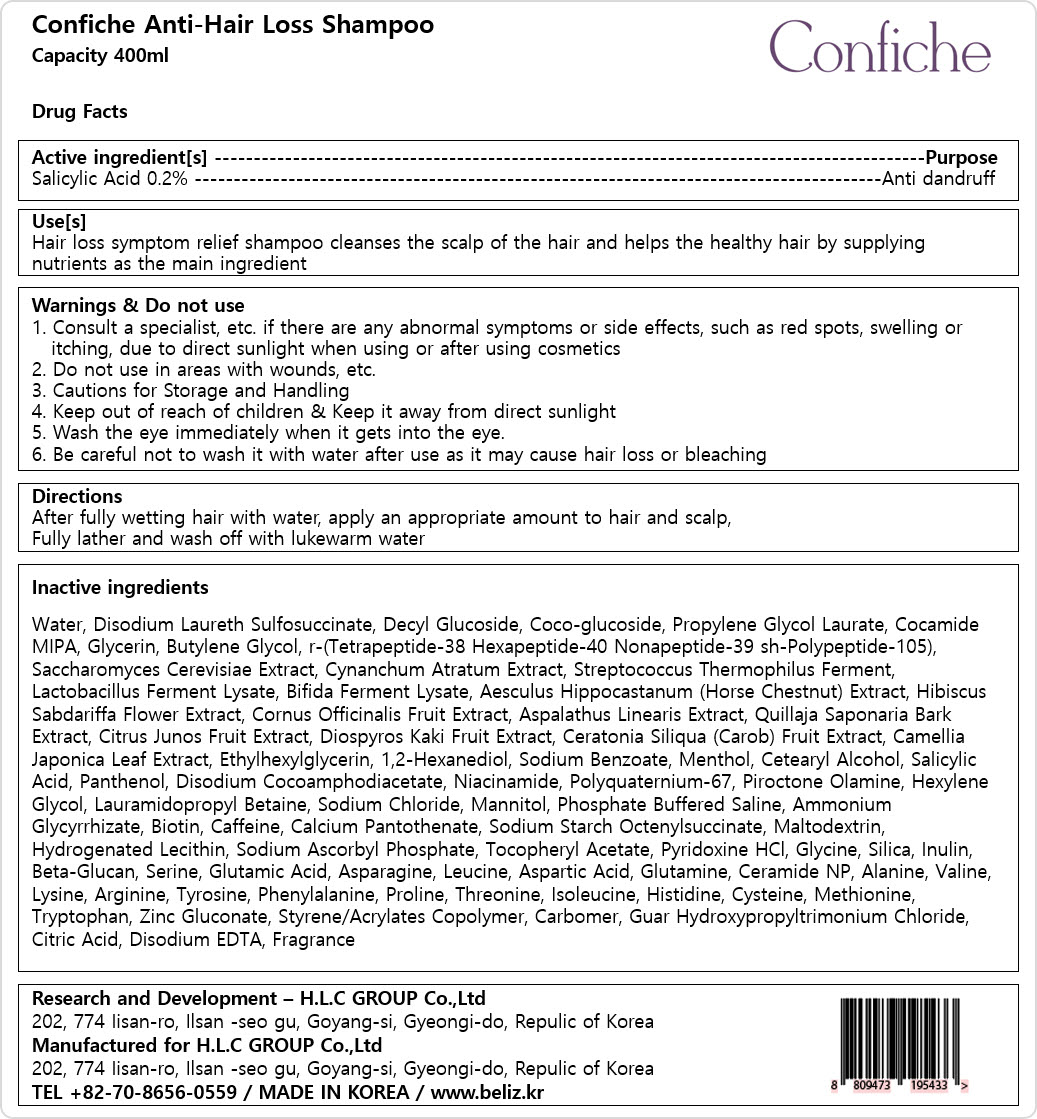
Uses
Hair loss symptom relief shampoo cleanses the scalp of the hair and helps the healthy hair by supplying nutrients as the main ingredient
Warnings
1. Consult a specialist, etc. if there are any abnormal symptoms or side effects, such as red spots, swelling or
itching, due to direct sunlight when using or after using cosmetics
2. Do not use in areas with wounds, etc.
3. Cautions for Storage and Handling
4. Keep out of reach of children & Keep it away from direct sunlight
5. Wash the eye immediately when it gets into the eye.
6. Be careful not to wash it with water after use as it may cause hair loss or bleaching
Warnings
Consult a specialist, etc. if there are any abnormal symptoms or side effects, such as red spots, swelling or
itching, due to direct sunlight when using or after using cosmetics
Directions
After fully wetting hair with water, apply an appropriate amount to hair and scalp,
Fully lather and wash off with lukewarm water
Inactive ingredients
Water, Disodium Laureth Sulfosuccinate, Decyl Glucoside, Coco-glucoside, Propylene Glycol Laurate, Cocamide
MIPA, Glycerin, Butylene Glycol, r-(Tetrapeptide-38 Hexapeptide-40 Nonapeptide-39 sh-Polypeptide-105),
Saccharomyces Cerevisiae Extract, Cynanchum Atratum Extract, Streptococcus Thermophilus Ferment,
Lactobacillus Ferment Lysate, Bifida Ferment Lysate, Aesculus Hippocastanum (Horse Chestnut) Extract, Hibiscus
Sabdariffa Flower Extract, Cornus Officinalis Fruit Extract, Aspalathus Linearis Extract, Quillaja Saponaria Bark
Extract, Citrus Junos Fruit Extract, Diospyros Kaki Fruit Extract, Ceratonia Siliqua (Carob) Fruit Extract, Camellia
Japonica Leaf Extract, Ethylhexylglycerin, 1,2-Hexanediol, Sodium Benzoate, Menthol, Cetearyl Alcohol, Salicylic
Acid, Panthenol, Disodium Cocoamphodiacetate, Niacinamide, Polyquaternium-67, Piroctone Olamine, Hexylene
Glycol, Lauramidopropyl Betaine, Sodium Chloride, Mannitol, Phosphate Buffered Saline, Ammonium
Glycyrrhizate, Biotin, Caffeine, Calcium Pantothenate, Sodium Starch Octenylsuccinate, Maltodextrin,
Hydrogenated Lecithin, Sodium Ascorbyl Phosphate, Tocopheryl Acetate, Pyridoxine HCl, Glycine, Silica, Inulin,
Beta-Glucan, Serine, Glutamic Acid, Asparagine, Leucine, Aspartic Acid, Glutamine, Ceramide NP, Alanine, Valine,
Lysine, Arginine, Tyrosine, Phenylalanine, Proline, Threonine, Isoleucine, Histidine, Cysteine, Methionine,
Tryptophan, Zinc Gluconate, Styrene/Acrylates Copolymer, Carbomer, Guar Hydroxypropyltrimonium Chloride,
Citric Acid, Disodium EDTA, Fragrance