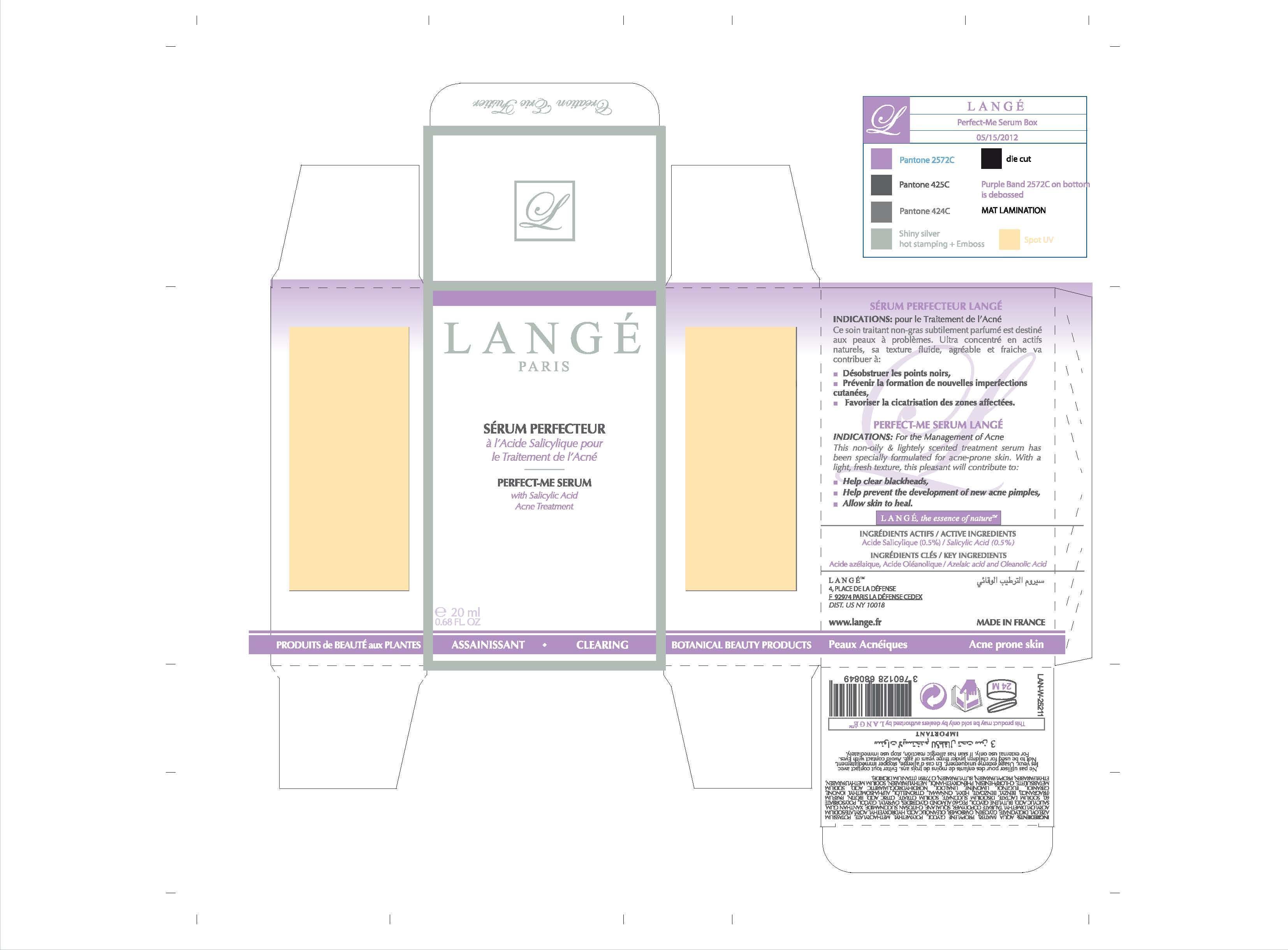
PERFECT-ME SERUM LANGE
INDICATIONS: For the Management of Acne
This non-oily and lightely scented treatment serum has been specially formulated for acne-prone skin. With a light, fresh texture, this pleasant will contribute to:
Help clear blackheads,
Help prevent the development of new acne pimples,
Allow skin to heal.
Not to be used for children under three years of age. Avoid contact with Eyes.
For external use only. If skin has allergic reaction, stop use immediately.
WATER
PROPYLENE GLYCOL
POLYMETHYL METHACRYLATE
POTASSIUM AZELOYL DIGLYCINATE
GLYCERIN
CARBOMER
OLEANOLIC ACID
HYDROXYETHYL ACRYLATE/SODIUM
ACRYLOYLDIMETHYLTAURATE
COPOLYMER
SQUALANE
CHITOSAN SUCCINAMIDE
XANTHAN GUM
SALICYLIC ACID
BUTYLENE GLYCOL
PEG-60 ALMOND GLYCERIDES
CAPRYLYL GLYCOL
POLYSORBATE 60
SODIUM LACTATE
DISODIUM SUCCINATE
SODIUM CITRATE
CITRIC ACID
BIOTIN
BENZYL BENZOATE
HEXYL CINNAMAL
CITRONELLOL
ALPHA-ISOMETHYL IONONE
GERANIOL
EUGENOL
LIMONENE
LINALOOL
NORDIHYDROGUAIARETIC ACID
SODIUM METABISULFITE
CHLORPHENESIN
PHENOXYETHANOL
METHYLPARABEN
SODIUM METHYLPARABEN
BUTYLPARABEN
ETHYLPARABEN
PROPYLPARABEN
ISOBUTYLPARABEN
CI 77891 (TITANIUM DIOXIDE)
PROPYLENE GLYCOL
POLYMETHYL METHACRYLATE
POTASSIUM AZELOYL DIGLYCINATE
GLYCERIN
CARBOMER
OLEANOLIC ACID
HYDROXYETHYL ACRYLATE/SODIUM
ACRYLOYLDIMETHYLTAURATE
COPOLYMER
SQUALANE
CHITOSAN SUCCINAMIDE
XANTHAN GUM
SALICYLIC ACID
BUTYLENE GLYCOL
PEG-60 ALMOND GLYCERIDES
CAPRYLYL GLYCOL
POLYSORBATE 60
SODIUM LACTATE
DISODIUM SUCCINATE
SODIUM CITRATE
CITRIC ACID
BIOTIN
BENZYL BENZOATE
HEXYL CINNAMAL
CITRONELLOL
ALPHA-ISOMETHYL IONONE
GERANIOL
EUGENOL
LIMONENE
LINALOOL
NORDIHYDROGUAIARETIC ACID
SODIUM METABISULFITE
CHLORPHENESIN
PHENOXYETHANOL
METHYLPARABEN
SODIUM METHYLPARABEN
BUTYLPARABEN
ETHYLPARABEN
PROPYLPARABEN
ISOBUTYLPARABEN
CI 77891 (TITANIUM DIOXIDE)