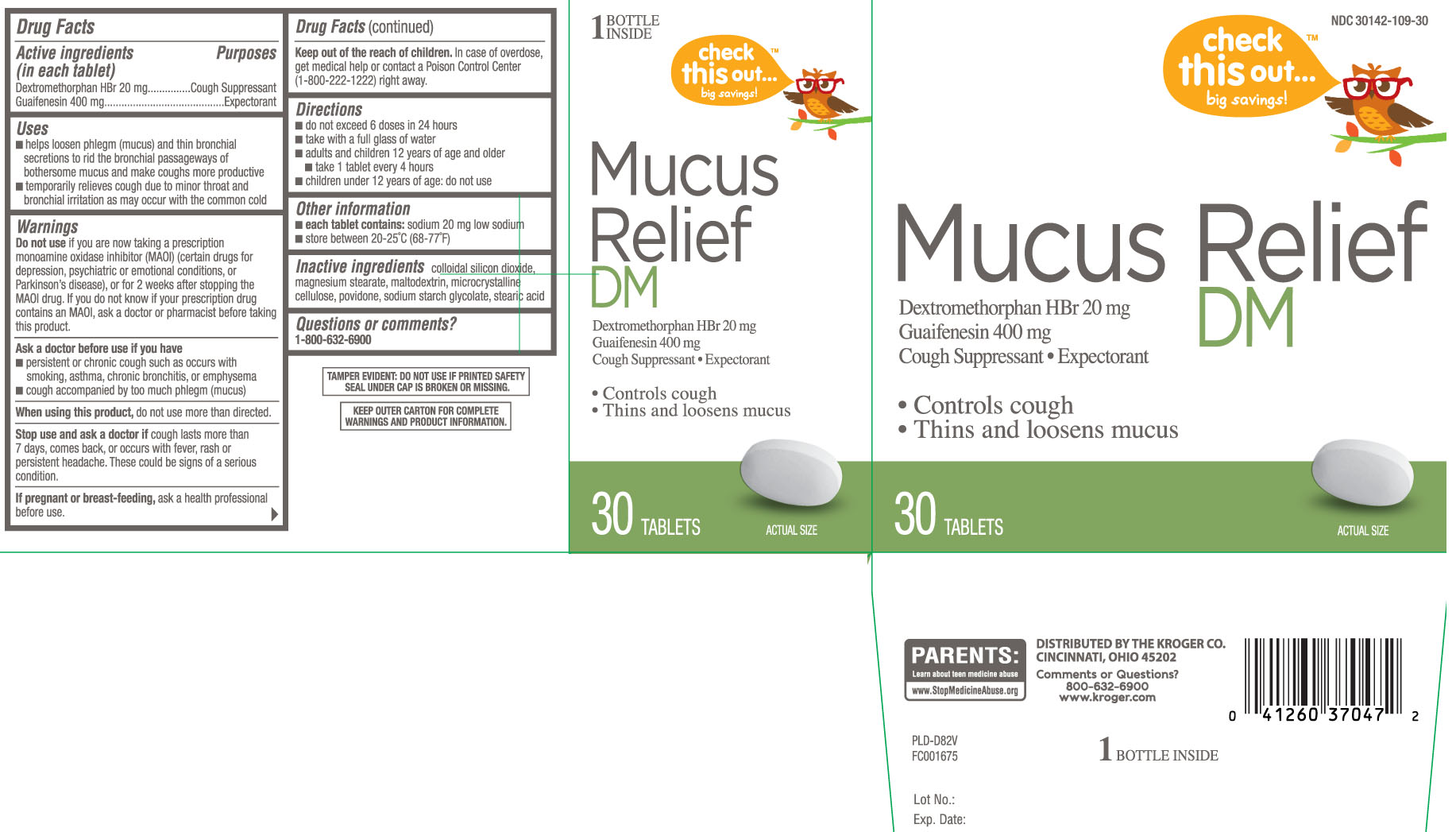
MUCUS RELIEF DM- dextromethorphan hydrobromide and guaifenesin tablet, coated
The Kroger Co.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with the common cold
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageway of bothersome mucus and make coughs more productive
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
- do not exceed 6 doses in 24 hours
- take with a full glass of water
- adults and children 12 years of age and older
- take 1 tablet every 4 hours
- children under 12 years of age: do not use
Inactive ingredients
colloidal silicon dioxide, magnesium stearate, maltodextrin, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
Principal Display Panel
Mucus Relief DM
Dextromethorphan HBr 20 mg
Guaifenesin 400 mg
Cough Suppressant • Expectorant
- Controls cough
- Thins and loosens mucus
TABLETS
DISTRIBUTED BY THE KROGER CO., CINCINNATI, OHIO 45202
Comments or Questions? 800-632-6900 www.kroger.com
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
| MUCUS RELIEF DM
dextromethorphan hydrobromide and guaifenesin tablet, coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - The Kroger Co. (006999528) |