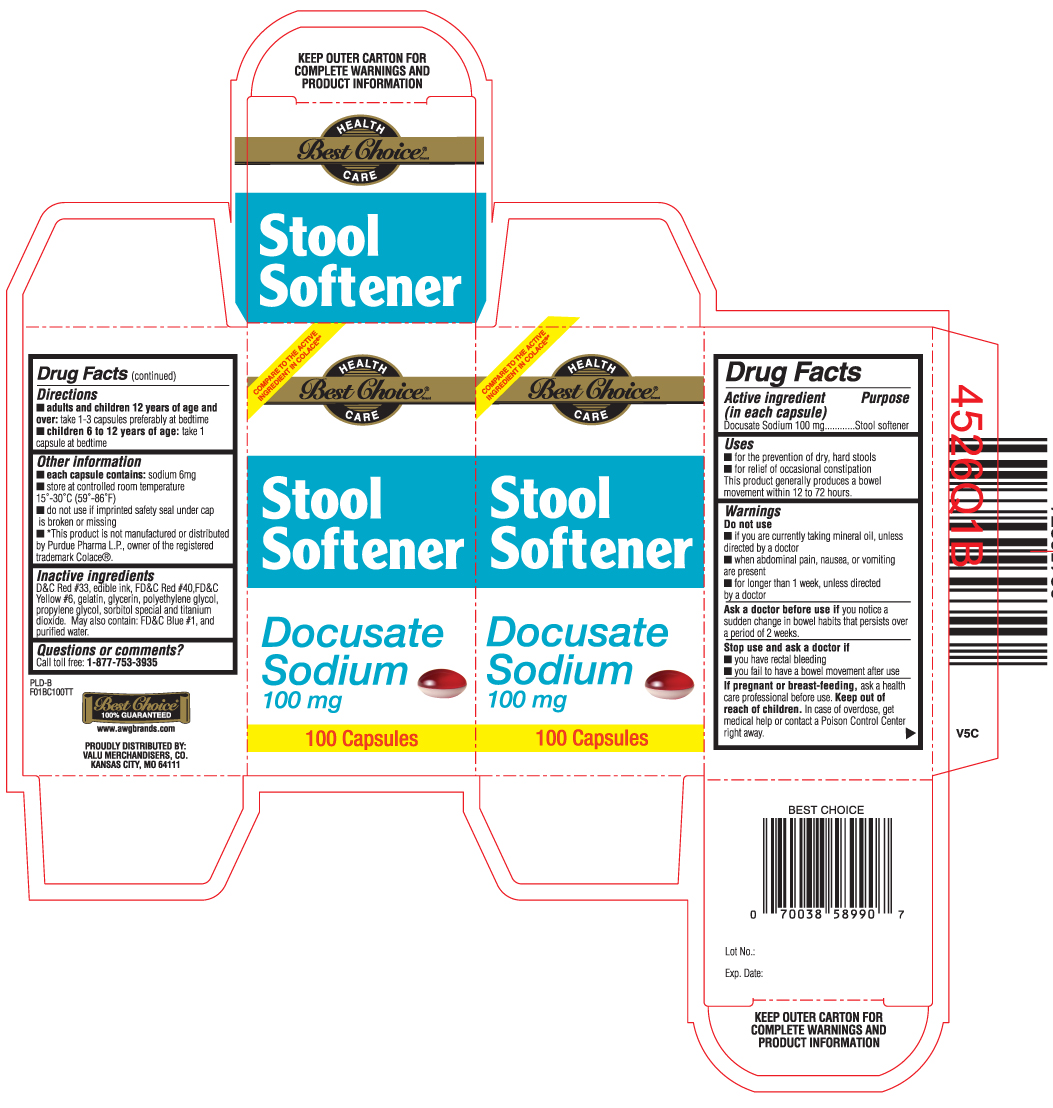
Uses
- for the prevention of dry, hard stools
- for relief of occasional constipation. This product generally produces a bowel movement within 12 to 72 hours.
Warnings - Do not use
- if you are currently taking mineral oil, unless directed by a doctor
- when abdominal pain, nausea or vomiting are present
- for longer than 1 week, unless directed by a doctor
Directions
-
adults and children 12 years of age and over: take 1- 3 capsules, preferably at bedtime
- children 6 to 12 years of age: take 1 capsule at bedtime
Other information
- each capsule contains: sodium 6 mg
- store at controlled room temperature 15o - 30o C (59o- 86o F)
- do not use if imprinted safety seal under cap is broken or missing.
- *This product is not manufactured or distributed by Purdue Pharma L.P., owner of the registered trademark Colace®
Inactive Ingredients
D&C Red #33, FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, sorbitol special and titanium dioxide. May also contain: FD&C blue #1 and purified water.