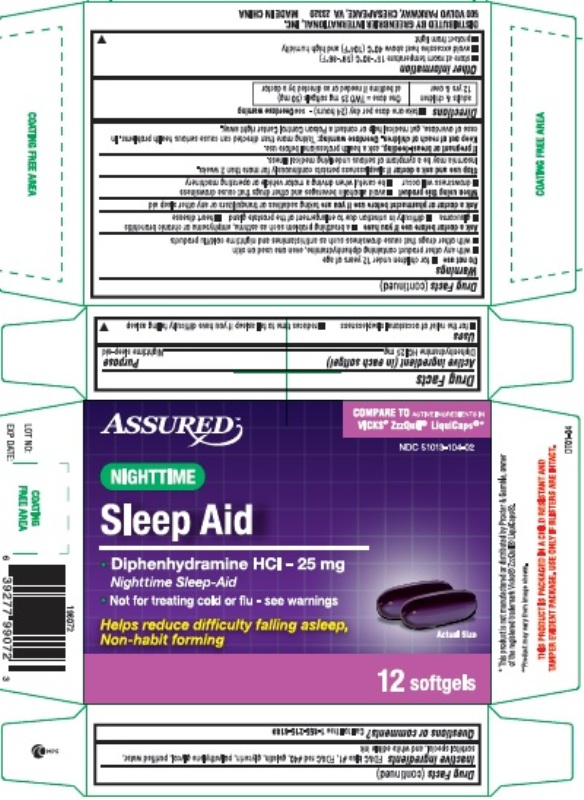
Uses
- for the relief of occasional sleeplessness
- reduces time to fall asleep if you have difficulty falling asleep
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- with other drugs that cause drowsiness such as antihistamines and nighttime cold/flu products
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
- heart disease
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers or any other sleep aid
When using this product
- avoid alcoholic beverages and other drugs that cause drowsiness
- drowsiness will occur
- be careful when driving a motor vehicle or operating machinery
Directions
- take only one dose per day (24 hours) - see Overdose warning
| adults and children 12 years and over | ONE dose = TWO 25 mg softgels (50 mg) at bed time if needed or as directed by a doctor |
Other information
- store at 15-30° C (59-86° F)
- avoid excessive heat above 40° C (104° F) and high humidity
- protect from light
Inactive ingredients
FD&C blue #1, FD&C red #40, gelatin, glycerin, polyethylene glycol, purified water, sorbitol special, and white edible ink
PRINCIPAL DISPLAY PANEL
Nighttime Sleep-Aid
Diphenhydramine HCl, 25 mg
Compare to Vicks® ZzzQuil® LiquiCaps® Active Ingredient*
12 Softgels
NDC 51013-104-02
Distributed by: Greenbrier International, Inc.
500 Volvo Parkway, Chesapeake, VA 23320
*This product is not manufactured or distributed by Procter & Gamble, owner of the registered trademark Vicks® ZzzQuil® LiquiCaps®.