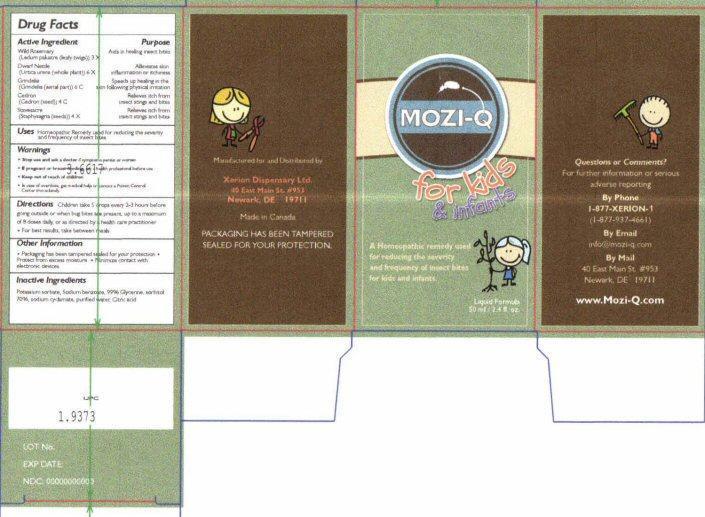
Drug Facts Active Ingredients
Wild Rosemary (Ledum palustre (leafy twigs)) 3 X
Dwarf Nettle (Urtica urens (whole plant)) 6 X
Grindelia (Grindelia (aerial part)) 6 C
Cedron (Cedron (seed)) 4 C
Stavesacre (Staphysagria (seeds)) 4 X
Purpose
Aids in healing insect bites
Alleviates skin inflammation or itchiness
Speeds up healing in the skin following physical irritation
Relieves itch from insect stings and bites
Warnings
- Stop use and ask a doctor if symptoms persist or worsen
- If pregnant or breast-feeding ask a health professional before use
- In case of overdose, get medical help or contact a Poison Control Center immediately
Directions
Children take 5 drops every 2-3 hours before going outside or when bug bites are present, up to a maxium of 8 doses daily, or as directed by a health care practitioner
- For best results, take between meals
Other Information
- Packaging has been tampered sealed for your protection
- Protect from excessive moisture
- Minimize contact with electronic devices
Inactive Ingredients
Potassium sorbate, Sodium benzoate, 99% Glycerine, sorbitol 70%, sodium cyclamate, purified water, citric acid
Product Label MOZI-Q for kids & infants
MOZI-Q for kids and infants
A Homeopathic remedy used for reducing the severity and frequency of insect bites for kids and infants
Liquid Formula 50 ml / 2.4 fl. oz.
Questions or Comments?
For further information or serious adverse reporting
By Phone 1-877-XERION-1 (1-877-937-4661)
By Email info@mozi-q.com
By Mail 40 East Main St # 953 Newark, DE 19711
www.Mozi-Q.com
Manufactured for and Distributed by
Xerion Dispensary Ltd.
40 East Main St #953
Newark, DE 19711
Made in Canada
PACKAGING HAS BEEN TAMPERED SEALED FOR YOUR PROTECTION
LOT No.
EXP DATE:
NDC 00000000000